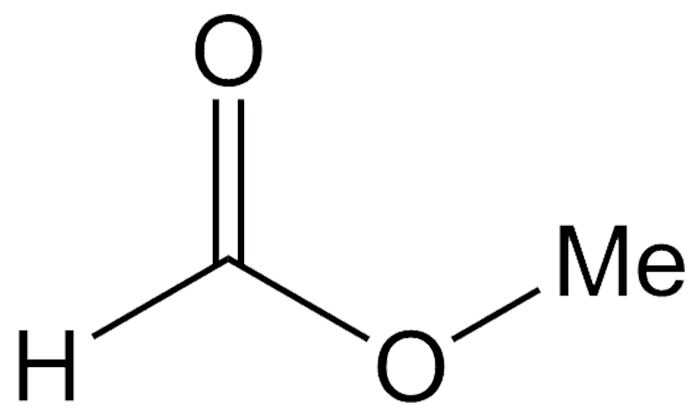
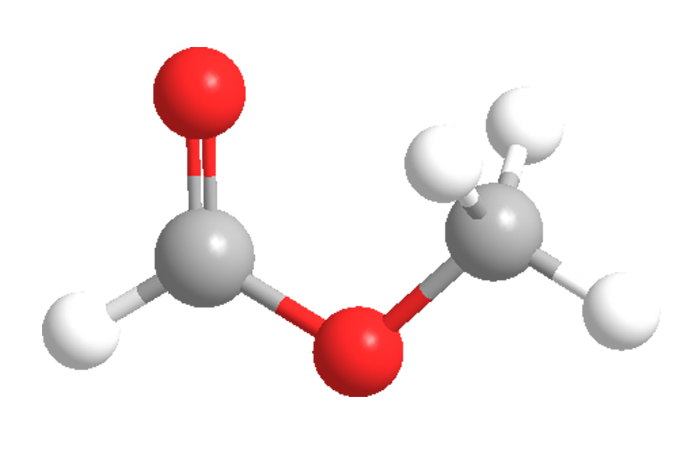
Methyl formate, a clear, volatile liquid, is the simplest carboxylate ester. It can be made in the lab by the acid-catalyzed esterification of formic acid and methanol. Industrially, it is produced by the base-catalyzed reaction of methanol and carbon monoxide.
The industrial uses of methyl formate include the manufacture of other formic acid derivatives, as a blowing agent for foams, and as an agricultural fumigant. It was formerly used as a refrigerant as an alternative to sulfur dioxide even though it also poses a toxicity hazard.
Methyl formate has been used as a model ester to study the combustion mechanisms of much more complex biodiesel mixtures. Toward this end, E. A. Carter and co-workers at Princeton University recently performed calculations to predict the hydrogen abstraction kinetics of methyl formate. They found that the predicted rate constants differed significantly from those that were calculated in previous combustion kinetics models.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

