What molecule am I?
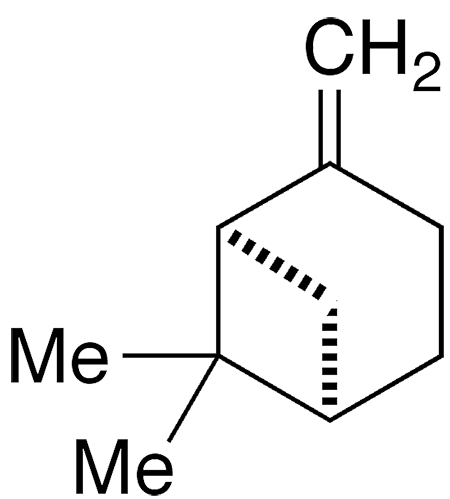
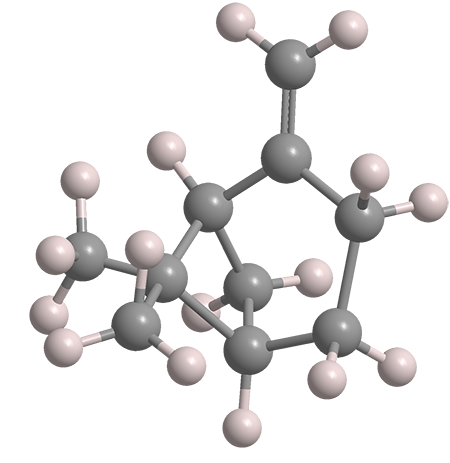
β-Pinene, a bicyclic monoterpene, is the second-most abundant constituent of the resins produced by pine trees and other conifers. The most abundant is its isomer α-pinene1, the Molecule of the Week for May 14, 2012. Both pinenes exist in nature as their (+)- and (–)-enantiomers. The images show the (+)-enantiomer of β-pinene.
The pinenes are the chief ingredients of turpentine2, a solvent that was in widespread use as a diluent and cleaner when oil-based paints were in their heyday. It is also used in varnishes and as a raw material for synthesizing useful organic compounds.
Turpentine was mentioned in the chemical literature as early as 1875 in US Patent 162,394, in which Archibald K. Lee of Galveston, TX, used it in an improved process to dissolve “asphaltum”, a soluble form of asphalt. In 1893, Walter E. Rohner of New York City was awarded US 498,961 for its use in a “wood-polishing compound”.
β-Pinene itself was first identified in 1896 by noted German chemist Adolf von Baeyer in an extensive paper on the origins of terpenes. Baeyer, who also developed syntheses of indigo, phenolphthalein, and fluorescein, was awarded the 1905 Nobel Prize in Chemistry.
Much more information about β-pinene can be found in ScienceDirect’s information page on the molecule.
1. CAS Reg. No. 80-56-8.
2. CAS Reg. No. 9005-90-7.
β-Pinene hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 3 | H226—Flammable liquid and vapor | |
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Aspiration hazard, category 1 | H304—May be fatal if swallowed and enters airways | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Sensitization, skin, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
Hirsutidin1 is an O-methylated cationic anthocyanidin that is usually present as its chloride2. It is a major component of the petals of the Madagascar periwinkle (Catharanthus roseus). Last October, Imran Kazmi at King Abdulaziz University (Jeddah, Saudi Arabia) and collaborators there and at other institutions in Saudi Arabia and India discovered that hirsutidin significantly reduces ethanol-induced stomach ulcers in rats.
Ochratoxins constitute a group of at least four mycotoxins found in some species of Aspergillus molds and Penicillium fungi. The most abundant member, ochratoxin A3 (OTA) is a common food contaminant; it is also frequently found in heating ducts and water-damaged houses. Noting that OTA is removed during the refining of edible oils, Xiaoyang Li, Yonghua Xiong, and colleagues at Nanchang University (Jiangxi, China) sought to determine the fate of the toxin when rapeseed oil is refined. Last November, they reported that 16% of the OTA is transferred to byproducts such as wastewater and soap stock; the other 84% is hydrolyzed to a ring-opened molecule, which is also a strong toxin that needs to be removed.
Selectfluor4 is a widely used fluorinating reagent developed and marketed by Air Products and Chemicals (Allentown, PA). It is synthesized in three steps from former Molecule of the Week DABCO. It is much safer and more convenient to use than fluorine gas. Last September, Ramazan Koçak, Bilal Nişancı, and co-workers at Ataturk University (Ezurum, Turkey) reported that Selectfluor (used in this case as an oxidant) and tetrabutylammonium bromide5 or chloride6 can combine to dihalogenate alkenes and alkynes without the use of metal catalysts or elemental halogens.
1. CAS Reg. No. 151776-57-7.
2. CAS Reg. No. 4092-66-4.
3. CAS Reg. No. 303-47-9.
4. CAS Reg. No. 140681-55-6; 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate).
5. CAS Reg. No. 1643-19-2.
6. CAS Reg. No. 1112-67-0.
Editor’s note
A 9-year-old boy in Massachusetts suggested turpentine as a Molecule of the Week. We explained to him that turpentine is a mixture, not a single molecule. But we applaud his initiative and decided to honor him by describing a key ingredient of turpentine.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
β-Pinene fast facts
| CAS Reg. No. | 127-91-3 |
| SciFinder nomenclature | Bicyclo[3.1.1]heptane, 6,6-dimethyl-2-methylene- |
| Empirical formula | C10H16 |
| Molar mass | 136.23 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 166 °C |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

