What molecule am I?
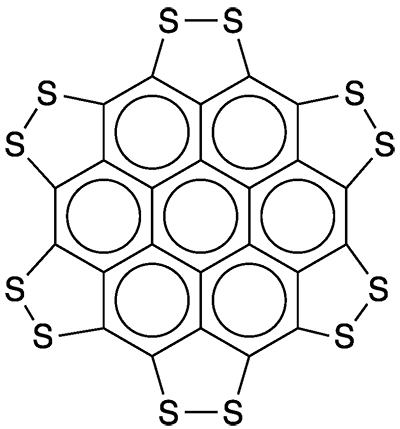
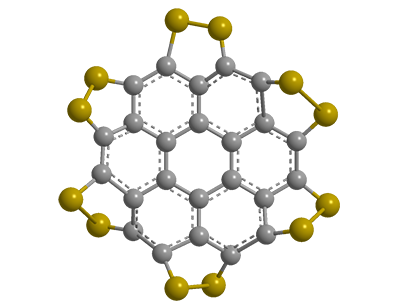
All organic chemists (and many others) know coronene as a highly symmetrical polycyclic aromatic hydrocarbon. It’s quite a useful compound, and a group of European chemists decided to increase its utility by adding sulfur atoms all around its periphery.
Klaus Müllen, Xinliang Feng, and 10 coauthors at the Technical University of Dresden (Germany), the Max Planck Institute for Polymer Research (Mainz, Germany), and the Polytechnic University of Milan (Italy), synthesized and measured the properties of persulfurated coronene. (Nomenclature purists will insist on calling it coroneno[1,12-cd:2,3-c′d′:4,5-c″d″:6,7-c‴d‴:8,9-c‴′d‴′:10,11-c‴″d‴″]hexakis[1,2]dithiole.)
Beginning with coronene, the researchers produced the persulfurated compound in four steps:
- chlorinating with AlCl3/ICl;
- thiobenzylating with benzyl mercaptan and base;
- displacing the benzyl groups with lithium; and
- removing the lithium atoms with acid under oxidizing conditions to form disulfide linkages.
The authors proceeded to obtain physical and spectral properties of their product, which they call a “sulflower”. Because of the compound’s low solubility in common organic solvents, however, they were unable to obtain an NMR spectrum or to grow single crystals. Despite these difficulties, they determined that the entire molecule is symmetrically hexagonal and coplanar.
Persulfurated coronene isn’t just a laboratory curiosity. The authors postulated that because of its high sulfur content and favorable redox behavior, their sulflower could work well in cathodes for lithium–sulfur batteries. Experimentation bore this out: Initial discharge and charge capacities of 424 and 496 mAh/g, respectively, reflected a high coulombic efficiency of 85%. This and other results showed that the performance of the new molecule compares favorably with other sulfur-based materials.
In December, C&EN named persulfurated coronene as one of its molecules of the year for 2017.
Persulfurated coronene fast facts
| CAS Reg. No. | 2073844-77-4 |
| Molar mass | 673.04 g/mol |
| Empirical formula | C24S12 |
| Appearance | Dark red powder |
| Melting point | >300 °C |
| Water solubility | Insoluble |
Persulfurated coronene hazard information
| GHS classification*: This is a new substance, and its hazards may not have been determined. |
*Globally Harmonized System of Classification and Labeling of Chemicals.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

