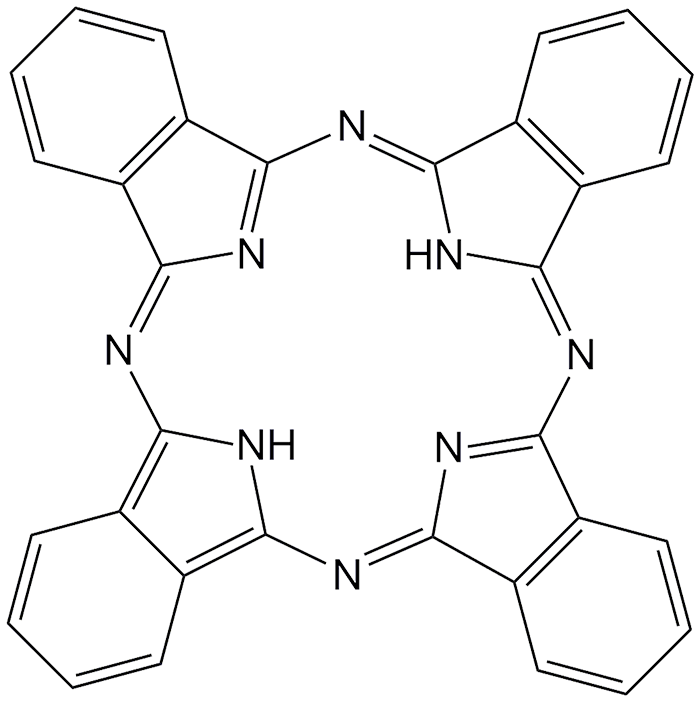
Phthalocyanine is an aromatic, intensely blue-green heterocyclic compound. Its structure is similar to that of porphine (the framework of molecules such as chlorophyll) with four fused benzene rings. It forms dye complexes with most metals in the periodic table, notably copper. A. Braun and J. Tcherniac reported the free molecule in 1907, but didn’t establish its structure. The copper form was made accidentally in 1927 when researchers were trying to make phthalonitrile.
Phthalocyanine dyes have been widely used for decades. But the molecule’s electronic properties make it potentially useful in the hi-tech world. Japanese researchers are devising ways to slow the tautomerization rate of phthalocyanine so that the molecule can exist in two electronically distinct states, corresponding to the binary system that has been the basis of computer technology. This development would give rise to “molecular memory” that could eventually be used in extremely fast computers. Phthalocyanine is also very compact: It may be able to store ≈13 terabytes of data on a 1-cm2 chip.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

