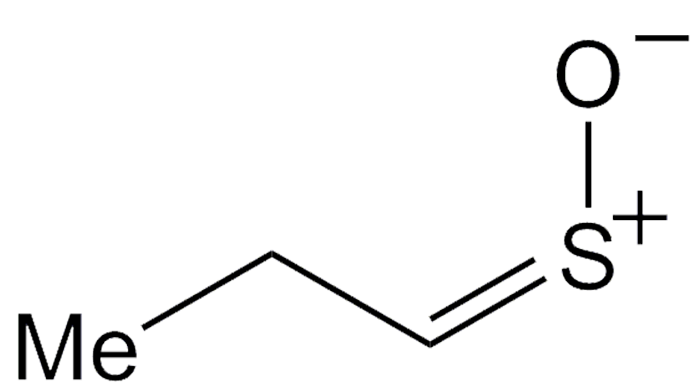
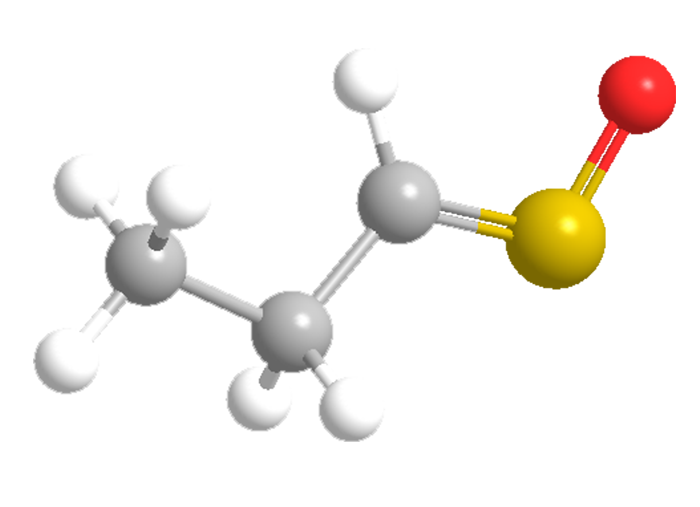
Why does slicing onions make you cry? The culprit is syn-propanethial S-oxide. Exposing allinase enzymes in onions to air generates 1-propenesulfenic acid. Another enzyme rearranges the acid to form this unusual volatile compound. When it hits your eyes, it stimulates the lachrymal glands to produce tears.
W. Niegisch and W. H. Stahl determined syn-propanethial S-oxide’s molecular formula in 1956. After many researchers attempted to establish its structure, E. Block and co-workers pinned it down in 1979 and showed that it has the syn, or Z, configuration. About 5% of the natural substance has the anti (E) configuration.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

