What molecule am I?
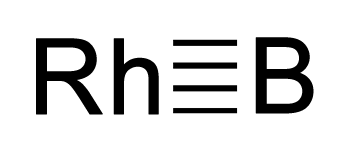
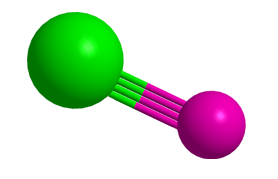
Rhodium boride (RhB) is a somewhat obscure inorganic compound that was first prepared in 1959 by B. Aronsson, J. Åselius, and E. Stenberg at the University of Uppsala (Sweden). They made it by heating a mixture of the component elements in an electric arc furnace. They noted that RhB forms orthorhombic crystals.
Between 2005 and 2009, several research groups investigated the hardness of binary compounds of transition metals with light elements (boron, carbon, nitrogen, and oxygen). Researchers found that many of these compounds are “superhard”; that is, they resist plastic and elastic deformations under pressures of 40 GPa or greater.
In a 2010 report, Julietta V. Rau and colleagues at the University of Rome, the National Research Council of Italy (Rome), and the University of Basilicata (Potenza, Italy) described the preparation and properties of RhB1.1 and IrB1.1 films. The authors used a pulsed laser deposition technique to create the films on a silica substrate from powdered metals and boron, with a slight excess of the latter.
The RhB1.1 and IrB1.1 films had Vickers hardness1 values of 44 ± 6 and 43 ± 5, respectively. Superhard materials are important in such industrial applications as wear-resistant coatings, cutting tools, and scratch-resistant surfaces.
RhB recently achieved a new distinction. While looking for the existence of a triple bond between a transition metal and boron in the gas phase, Lai-Sheng Wang and co-workers at Brown University (Providence, RI) ed a quadruple bond. The researchers chose rhodium because its sole natural isotope makes it easier to work with than multi-isotope atoms.
Experiments with photoelectron spectroscopy and theoretical calculations showed that the bond length in RhB is shorter than expected for a Rh–B triple bond, and its bond dissociation energy is greater. This result was confirmed when the calculated bond energy of RhB proved to be greater than that of its protonated form, RhBH+, which can only contain a triple bond.
The authors concluded that RhB must contain an unexpected quadruple bond. Although some researchers claim that C2 and other diatomic main-group species have quadruple bonding, this is the first such case to be confirmed. Just before the Wang paper was published, a research team in China reported a non-diatomic instance of main-group quadruple bonding in the BFe(CO)3– complex.
Rhodium boride is not an article of commerce; hence hazard information for it is not readily available.
1. A widely used hardness test. Diamond, the hardest substance known, has Vickers hardness values from 70 to 150 GPa.
Rhodium boride
fast facts
| CAS Reg. No. | 12523-58-9 |
| SciFinder nomenclature | Rhodium boride (RhB) |
| Empirical formula | BRh |
| Molar mass | 113.72 g/mol |
| Appearance | Dark gray solida |
| Melting point | 1140 ºC |
| Water solubility | Insolublea |
a. Based on typical properties of transition-metal borides

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

