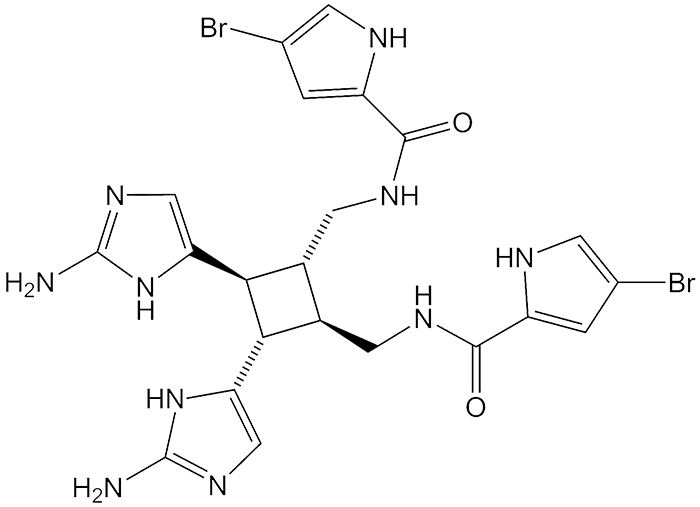
Sceptrin, an alkaloid isolated from marine sponges such as Agelas conifer, has an unusual structure with a core cyclobutane ring. In 2008, A. D. Rodríguez (University of Puerto Rico, San Juan), M. J. Lear (National University of Singapore), and J. J. La Clair (Xenobe Research Institute, San Diego) showed that sceptrin co-immunoprecipitates with MreB, an actin-like protein derived from Escherichia coli. This work was performed before sceptrin’s structure was known; the same researchers later established the structure.
Two years later, K. Vuori and colleagues at the Burnham Institute for Medical Research and the Scripps Research Institute (both in La Jolla, CA) reported that sceptrin inhibits cell motility in several cancer cell lines. It is not toxic to mice at concentrations twice those required for maximal inhibition.
In an interesting twist, C. Chen (University of Texas Southwestern Medical Center, Dallas), P. S. Baran (Scripps Research Institute), and A. L. Rheingold (University of California, San Diego) found in 2014 that sceptrin and two related sponge alkaloids don’t all have the same chirality pattern. One analogue, ageliferin, matches sceptrin’s chirality, whereas another alkaloid, massadine, has different absolute stereochemistry. It’s not clear why the parent sponge has varying biosynthesis sequences.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

