What molecule am I?
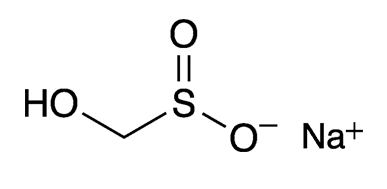
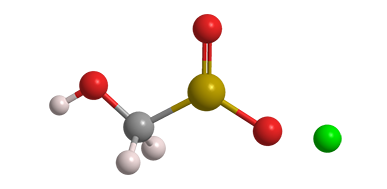
Sodium formaldehydesulfoxylate (SFS) is a long name for a rather small molecule. It also goes by the name sodium hydroxymethanesulfinate or, most commonly, its trade name Rongalite. It is usually marketed as the dihydrate.
SFS has a long history:
- In a short 1908 US patent assigned to Heyden Chemical Works (New York), Bruno R. Seifert and Otto W. Meves reduced formaldehyde and sulfur dioxide with zinc dust to a paste containing a zinc formaldehydesulfoxylate that could be converted to the sodium salt with sodium hydroxide or carbonate.
- In a 1922 article, Frederick W. Heyl and Frank E. Greer of Upjohn1 (Kalamazoo, MI) reported an improved synthesis of a method described in a 1913 German patent. A mixture of formaldehyde and sodium hydrogen sulfite is treated with a mixture of zinc dust and zinc oxide. A convoluted purification process leads to acceptably pure SFS.
- A 1935 patent by Frederick W. Binns at the Virginia Smelting Co.2 (Portland, ME) describes a similar synthesis that starts from zinc, sulfur dioxide, and formaldehyde.
Today SFS is manufactured from sodium dithionite (Na2S2O4) and formaldehyde. It was originally developed as a treatment for mercury poisoning (hence its pharmaceutical origin), but that was of limited value. Its current uses are in vat dyeing as a reducing agent, in redox polymerization initiator systems, and for aquarium water conditioning.
Earlier this month, Scientific Update, a Mayfield, UK–based firm that holds conferences and training courses for industrial chemists and chemical engineers, featured Rongalite as its Reagent of the Month. The article focuses on the uses of the reagent in organic chemical synthesis.
1. Upjohn is now part of Pfizer. In the May 1949 issue of an Upjohn house publication, Heyl was referred to as “the Father of Upjohn Research”.
2. Later based in West Norfolk, VA. See a 1949 ad in an ACS journal.
Sodium formaldehydesulfoxylate hazard information
| GHS classification*: acute toxicity, oral, category 4 | |
| H302—Harmful if swallowed | |
| GHS classification: germ cell mutagenicity, category 2 | |
| H341—Suspected of causing genetic defects | |
| GHS classification: reproductive toxicity, category 2 | |
| H361—Suspected of damaging fertility or the unborn child | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Sodium formaldehydesulfoxylate
fast facts
| CAS Reg. No. | 149-44-0 |
| Empirical formula | C3NaO3S |
| Molar mass | 118.10 g/mol |
| Appearance | Colorless crystals |
| Melting point | 64.5 ºCa |
| Water solubility | ≈600 g/La |
aData for the dihydrate.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

