What molecule am I?
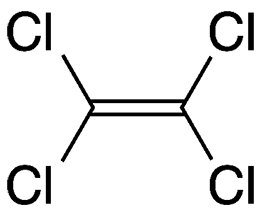
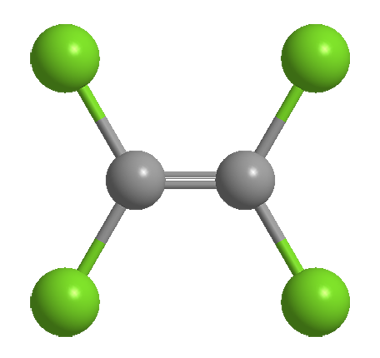
Tetrachloroethylene, often called perchloroethylene (PCE or “perc”), is a colorless, nonflammable liquid that is widely used for dry cleaning—which, of course, is not actually dry. It is not abbreviated as TCE because that is conventionally used for trichloroethylene.
In 1821, legendary chemist Michael Faraday discovered how to make PCE by heating hexachloroethane until it decomposed, with molecular chlorine as the byproduct. This process led to today’s wide variety of production methods in which light hydrocarbons or chlorohydrocarbons are heated in the presence of chlorine, with or without a catalyst, to yield a combination of PCE and many other chlorocarbons. The crude mixture must be separated by distillation
In addition to dry cleaning, PCE is used industrially as a solvent, degreaser, refrigerant, and starting material for fluorocarbon production. Its use in dry cleaning has declined precipitously in the past 40 years because of improved solvent recycling systems and worker health concerns (it is a probable carcinogen). Despite this downturn, its overall worldwide production is slowly increasing.
Potential PCE replacements for dry cleaning include 1-bromopropane, silicone fluids, and liquified carbon dioxide, all of which have health, environmental, or economic drawbacks. So when you send your holiday finery out for cleaning, it’s most likely that it will be treated with PCE.
MOTW update: June 26, 2023
Tetrachloroethylene is also known as perchloroethylene or “perc”, it is a nonflammable solvent widely used in dry cleaning. It is also a suspected carcinogen that has come under scrutiny by the US Environmental Protection Agency.
This month, EPA announced that it is proceeding to restrict the use of tetrachloroethylene. It proposed a rule to phase it out from all consumer uses in 2 years and from dry-cleaning uses within 10 years. This was after a 2020 agency finding that the solvent poses health risks, such as neurological, kidney, liver, and immunological effects.
Tetrachloroethylene
| CAS Reg. No. | 127-18-4 |
| Molar mass | 165.83 g/mol |
| Formula | C2Cl4 |
| Appearance | Colorless liquid |
| Boiling point | 121 ºC |
| Water solubility | 15 mg/L |
MOTW update:
January 22, 2018
Previous environmentally problematic Molecules of the Week tetrachloroethylene, N-methyl-2-pyrrolidone, and hexabromocyclododecane are in the news again. They are among the chemicals in the “First 10” list for reevaluation under the revised Toxic Substances Control Act of 2016. But last month the Trump administration put additional restrictions of the substances on hold, with no set date for implementation.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

