What molecule am I?

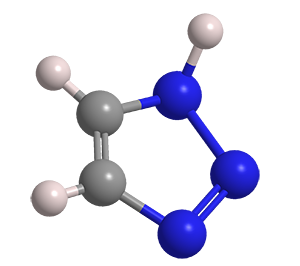
Triazoles are a family of five-membered rings that contain three nitrogen atoms and two double bonds. The four triazole isomers (1H-1,2,3-, 2H-1,2,3-, 1H-1,2,4-, and 2H-1,2,4-) differ by the arrangement of the nitrogen atoms and the locations of their three hydrogen atoms. All of the triazoles are planar and aromatic.
1H-1,2,3-Triazole, like all triazoles, is highly soluble in water. In aqueous solution it tautomerizes to its 2H-isomer with a 1H/2H ratio of ≈1:2. Both isomers have essentially the same melting and boiling points, making them very difficult to separate from each other.
In 1910, German chemists Otto Dimroth* and Gustav Fester synthesized 1H-1,2,3-triazole by heating a solution of hydrazoic acid (HN3) and acetylene at 100 ºC for 70 h. Sodium azide can be used in place of HN3 if the solution is acidified. Dimroth is best known for his discovery of the eponymous rearrangement of amine-substituted 1,2,3-triazoles, in which the substituent nitrogen atom and its nearest ring nitrogen atom exchange places.
The 1,2,3-triazole moiety is considered to be a pharmacophore in that it can interact with specific biological targets. It is a component of such pharmaceuticals as the cephalosporin antibiotic cefatrizine; tazobactam, which broadens the spectrum of certain antibiotics; and carboxyamidotriazole, a calcium channel blocker that may be useful to combat cancer.
1H-1,2,3-Triazole hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
MOTW update
Baricitinib was the Molecule of the Week for March 2, 2020. It is a janus kinase inhibitor that initially showed promise against rheumatoid arthritis. This past February, researchers reported that it may limit the effects of COVID-19 by attacking a specific enzyme in the virus. On November 19, the US Food and Drug Administration issued an emergency use authorization for the combination and remdesivir (MOTW for April 6, 2020) “for the treatment of suspected or laboratory-confirmed COVID-19 in hospitalized adults and pediatric patients two years of age or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation.”
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
1H-1,2,3-Triazole fast facts
| CAS Reg. No. | 288-36-8 |
| SciFinder nomenclature | 1H-1,2,3-Triazole |
| Empirical formula | C2H3N3 |
| Molar mass | 69.07 g/mol |
| Appearance | Colorless to yellow liquid |
| Melting point | 23–25 ºC |
| Boiling point | 203 ºC |
| Water solubility | Highly soluble |
MOTW update:
September 19, 2022
1H-1,2,3-Triazole is one of the four aromatic heterocyclic compounds with two carbon atoms, three nitrogen atoms, and two double bonds. In aqueous solution, it is in equilibrium with its 2H-tautomer.
This month, Xiaowei Song, Yifan Meng, and Richard N. Zare* at Stanford University (CA) reported that the 1,2,3-triazoles play a key role in converting carbon dioxide to formic acid. The authors sprayed microdroplets of an aqueous 1,2,3-triazole solution into a gas-phase reactor containing CO2; the droplets captured CO2 and reduced it to formic acid at the gas–liquid interface. The process was optimized to achieve yields of >80%.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

