What molecule am I?
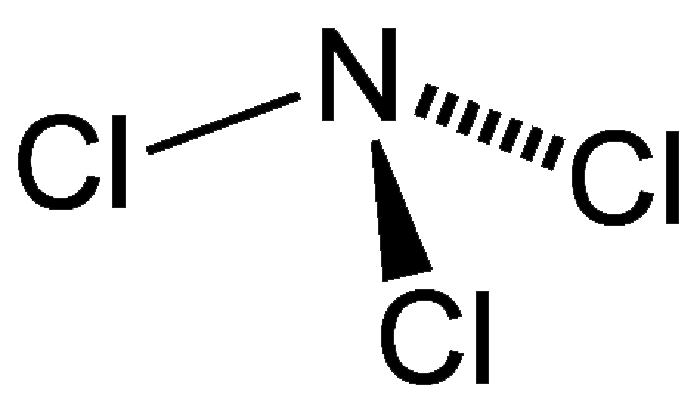
Trichloramine (NCl3), also known as nitrogen chloride or trichloride, is a toxic, explosive, nasty-smelling oily liquid with a boiling point of 71 ºC. It is unstable in air and rapidly decomposes in water.
The earliest reported synthesis of NCl3 (Pierre Louis Dulong, 1813) was the reaction of molecular chlorine or sodium hypochlorite with ammonium salts. It can also be prepared from ammonia and chlorine and by the electrolysis of aqueous ammonium chloride.
NCl3’s primary commercial use was bleaching flour, but this use was banned in the United States in the late 1940s. It had been discovered in 1947 that eating bread made from NCl3-contaminated flour caused severe neurological disorders in humans and animals.
NCl3 is most famous (or infamous) for being the product of the reaction of chlorine in swimming pools with urea in urine or sweat. It is much more responsible for pools’ “chlorine smell” than chlorine itself.
In an article on disinfection byproducts in swimming pools related to the Rio de Janeiro Olympic Games, Celia Henry Arnaud discusses NCl3 in connection with professional swimmers’ habit of intentionally peeing in pools. NCl3 can not only cause respiratory problems in swimmers, but it also can corrode metals in and around pools.
Mr. Phelps: Your mission, should you choose to accept it, is to discontinue this practice and urge other Olympians to do the same.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

