What molecule am I?

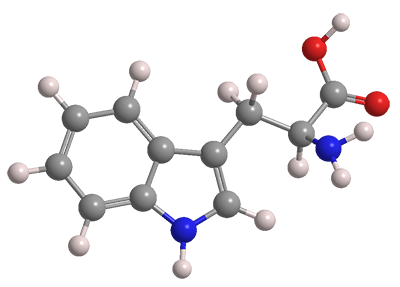
L-Tryptophan is one of nine essential amino acids for humans—“essential” in that the body cannot synthesize them, so they must be part of the diet. Fortunately, tryptophan is a constituent of many common foods, especially ones that are high in protein.
In 1901, British biochemists Frederick Gowland Hopkins and Sydney W. Cole discovered tryptophan in the milk derivative casein. (In 1929, Hopkins was a co-winner of the Nobel Prize in Physiology or Medicine for the discovery of vitamins.) The first lab synthesis of tryptophan was reported by German chemist/pharmacologist Alexander Ellinger in 1906.
In the body, tryptophan is a biochemical precursor to serotonin and melatonin, a neurotransmitter and a hormone, respectively. Low serotonin levels are associated with depression, which has prompted the marketing of tryptophan as an over-the-counter depression treatment. The scientific evidence for this use, however, is sketchy. Most commercial tryptophan is produced by microbial fermentation.
Legend has it that melatonin produced from large amounts of tryptophan in turkey meat causes drowsiness after a Thanksgiving dinner. It turns out, however, that turkey contains no more tryptophan than other types of meat. It’s more likely that the consumption of copious quantities of all kinds of food prompts our post–turkey feast naps.
Originally the Molecule of the Week for November 20, 2006
MOTW update: February 8, 2021
L-Tryptophan is an essential amino acid that must be part of the human diet. Among other functions, it enables the body to synthesize serotonin and melatonin. Recently, tryptophan appeared in two biochemical studies.
In one, researchers at Pennsylvania State University (University Park) and the Massachusetts Institute of Technology (Cambridge) discovered that the enzyme tryptophan 2C methyltransferase, which catalyzes the addition of a methyl group to tryptophan’s indole ring in the synthesis of the antibiotic thiostrepton, does not use a radical mechanism as do most enzymes in its class. Instead, a carboxylate group in the enzyme acts as a general base to deprotonate the tryptophan substrate.
In the second study, A. Keith Dunker at Indiana University (Bloomington) and Klára Hlouchová at Charles University (Prague) collaborated in a quest to discover how life on Earth began. They hypothesized that inherently disordered proteins (IDPs), ones that don’t hold to a specific conformation, but move around, were involved early in the process. They believe that the unstructured proteins formed by IDPs were eventually augmented by the later-forming aromatic amino acids tryptophan and tyrosine, which are larger and give proteins a greater degree of structure. They tested their ideas by replacing the aromatic amino acids in a key enzyme with leucine; the modified, less structured enzyme retained some activity, but ultimately failed to perform as efficiently as the original.
L-Tryptophan fast facts
| CAS Reg. No. | 73-22-3 |
| Molar mass | 204.23 g/mol |
| Formula | C11H12N2O2 |
| Appearance | White crystals or powder |
| Melting point | ≈290 ºC (dec.) |
| Water solubility | 11.4 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

