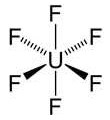
Uranium hexafluoride is a white-to-gray solid made by treating any of several uranium-containing materials, including metallic uranium, UC2, UCl5, and UF4, with molecular fluorine. It is covalently bonded, as would be expected from its low sublimation temperature of 56.5 °C. Its main use is separating uranium isotopes. UF6 is stable in dry air but reacts vigorously with water.
MOTW update:
November 14, 2022
Uranium hexafluoride1 (UF6) is a covalent compound used primarily for separating uranium isotopes. In a recent article, Kirk Peterson, Puru Jena, Kit H. Bowen, and colleagues at Johns Hopkins University (Baltimore), Washington State University (Pullman), and Virginia Commonwealth University (Richmond) used photoelectron spectroscopy and quantum chemistry to demonstrate the degree to which gold can act as a surrogate to fluorine in UF6. They found that, unlike UF6, UAu6 shows strong ligand–ligand (i.e., Au–Au) interactions that result in three low-lying isomers with 3-D or quasi-2-D structures.
1. CAS Reg. No. 7783-81-5.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

