What molecule am I?

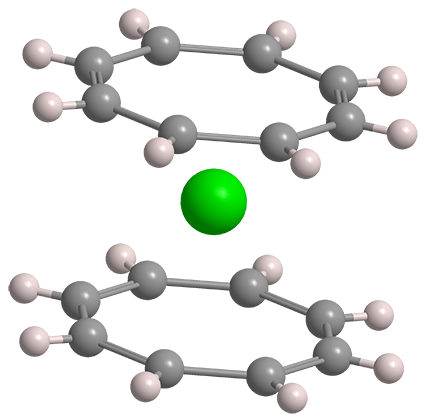
Ever since the discovery of ferrocene in the 1940s, hundreds of “sandwich” compounds, in which a metal atom is “sandwiched” between two arene rings, have been synthesized. In 1968, Andrew Streitweiser, Jr.,* and Ulrich Mueller-Westerhoff at the University of California, Berkeley, advanced the field by preparing uranocene, an actinide metal sandwiched between two cyclic polyolefins.
In contrast to ferrocene with its cyclopentadienide (Cp) rings, the coordinating molecule in uranocene is the dianion of cyclooctatetraene (COT). Streitweiser and Mueller-Westerhoff treated COT with elemental potassium to make the dianion and then added uranium tetrachloride at 0 ºC to produce uranocene, a green crystalline solid. COT has a folded structure; but its dianion is planar, making it a suitable “bread” for the sandwich.
Uranocene is stable to water, acids, bases, and moderate heating—but it ignites spontaneously in air. In ferrocene, the Cp rings’ six π electrons interact with the 3d orbitals in iron. In uranocene, uranium’s 6d orbitals similarly combine with the COT’s 10 π electrons; but uranium 5f orbitals are also involved, contributing to the molecule’s stability.
In 2004, Ditmar Seyferth at MIT wrote a comprehensive review of organometallic compounds that contain f-orbital elements.
Uranocene hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 1 | H300—Fatal if swallowed | |
| Acute toxicity, inhalation, category 1 | H330—Fatal if inhaled | |
| Specific target organ toxicity, repeated exposure, category 2 | H373—Causes damage to organs through prolonged or repeated exposure | |
| Hazardous to the aquatic environment, long-term hazard, category 2 | H411—Hazardous to the aquatic environment, long-term hazard | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
MOTW update
Ibogaine was the Molecule of the Week for June 16, 2014. It is a hallucinogenic, neurotoxic indole alkaloid that is successful in reducing withdrawal effects from abused drugs such as alcohol, nicotine, and methamphetamine. But ibogaine’s toxicity, especially its tendency to induce cardiac arrhythmia, led researchers to investigate safer efficacious analogues. David E. Olson and co-workers at the University of California, Davis, have synthesized the simpler molecule tabernanthalog that does not cause hallucinations in mice, nor does it inhibit the potassium channels linked to ibogaine’s cardiotoxicity. In rodent experiments, tabernanthalog reduced alcohol- and heroin-seeking behavior and had antidepressant effects.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Uranocene fast facts
| CAS Reg. No. | 11079-26-8 |
| SciFinder nomenclature | Uranium, bis(η8-1,3,5,7-cyclooctatetraene) coordination compound |
| Empirical formula | C16H16U |
| Molar mass | 446.33 g/mol |
| Appearance | Green crystals |
| Melting point | 180 ºC (subl) (4 Pa) |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

