What molecule am I?

Vanadium dioxide [vanadium(IV) oxide, (VO2)] is one of four relatively common oxides of vanadium. Others are
- vanadium monoxide [vanadium(II) oxide, VO];
- vanadium sesquioxide or trioxide [vanadium(III) oxide, V2O3]; and
- vanadium pentoxide [vanadium(V) oxide, V2O5].
In addition, several mixed vanadium oxides exist, many in nature. Examples are V3O7 (VO2 + V2O5) and V4O7 (2VO2 + V2O3). The vast range of vanadium oxides differ widely in terms of color, crystal structure, and chemical properties.
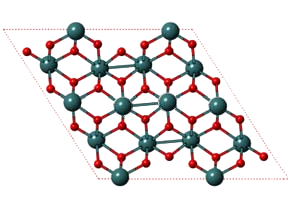
VO2 itself is sometimes expressed as V2O4, V4O8, or other VnO2n formulas, depending on its crystalline symmetry. At temperatures less than 67 ºC, VO2 has a monoclinic crystal structure (shown). When heated to more than 67 ºC, it transitions to a tetragonal structure. At the same temperature, crystalline VO2 converts from an electrical insulator to a conductor.
But wait, there’s more: In 2013, a team led by Masaki Nakano at the RIKEN Center for Emergent Matter Science (Wako) and Tohoku University (both in Japan) built on the insulator–conductor phenomenon to affect light transmission through VO2 glass. It had previously been shown that low-temperature (<30 ºC) VO2 glass is transparent to infrared (IR) radiation, but at >60 ºC, it reflects IR light. Nakano et al. accomplished the same result by applying an external voltage to a thin film of VO2. This discovery is the basis of new field-effect transistors.
And still more: In 2017, Olivier Delaire at the Oak Ridge National Laboratory (TN) and Duke University (Durham, NC); Junqiao Wu at the University of California, Berkeley, and Lawrence Berkeley National Laboratory; and colleagues worldwide showed that VO2 violates the venerable (1853) Wiedemann–Franz law, which holds that the ratio of the electronic component of the thermal conductivity of a metal to its electrical conductivity is proportional to the metal’s temperature. At and below the insulator–conductor transition temperature, the electronic thermal conductivity is anomalously low, which, the authors state, “is a signature of the absence of quasiparticles in a strongly correlated electron fluid where heat and charge diffuse independently."
What will scientists discover next about VO2?
Vanadium dioxide hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Safety data sheets vary widely; some state that VO2 is not classified or is not a hazardous substance.
**Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
Vanadium dioxide
fast facts
| CAS Reg. No. | 12036-21-4 |
| SciFinder nomenclature | Vanadium oxide (VO2) |
| Empirical formula | O2V |
| Molar mass | 82.94 g/mol |
| Appearance | Dark blue, black, or gray crystals or powder |
| Melting point | 1967 ºC |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

