FOR IMMEDIATE RELEASE | March 20, 2022
Giving the cold shoulder to crunchy ice cream — with a dash of cellulose
Note to journalists: Please report that this research will be presented at a meeting of the American Chemical Society.
A media briefing on this topic is available at www.acs.org/ACSSpring2022Briefings.
SAN DIEGO, March 20, 2022 — Ice cream can be a culinary delight, except when it gets unpleasantly crunchy because ice crystals have grown in it. Today, scientists report that a form of cellulose obtained from plants can be added to the tasty treat to stop crystals cold — and the additive works better than currently used ice growth inhibitors in the face of temperature fluctuations. The findings could be extended to the preservation of other frozen foods and perhaps donated organs and tissues.
The researchers will present their results today at the spring meeting of the American Chemical Society (ACS). ACS Spring 2022 is a hybrid meeting being held virtually and in-person March 20-24, with on-demand access available March 21-April 8. The meeting features more than 12,000 presentations on a wide range of science topics.
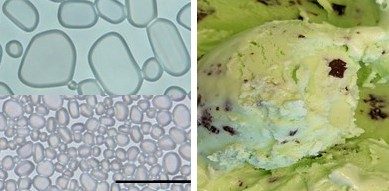
Freshly made ice cream contains tiny ice crystals. But during storage and transport, the ice melts and regrows. During this recrystallization process, smaller crystals melt, and the water diffuses to join larger ones, causing them to grow, says Tao Wu, Ph.D., the project’s principal investigator. If the ice crystals become bigger than 50 micrometers — or roughly the diameter of a hair — the dessert takes on a grainy, icy texture that reduces consumer appeal, Wu says. “Controlling the formation and growth of ice crystals is thus the key to obtaining high-quality frozen foods.”
One fix would be to copy nature’s solution: “Some fish, insects and plants can survive in sub-zero temperatures because they produce antifreeze proteins that fight the growth of ice crystals,” Wu says. But antifreeze proteins are costlier than gold and limited in supply, so they’re not practical to add to ice cream. Polysaccharides such as guar gum or locust bean gum are used instead. “But these stabilizers are not very effective,” Wu notes. “Their performance is influenced by many factors, including storage temperature and time, and the composition and concentration of other ingredients. This means they sometimes work in one product but not in another.” In addition, their mechanism of action is uncertain. Wu wanted to clarify how they work and develop better alternatives.
Although Wu didn’t use antifreeze proteins in the study, he drew inspiration from them. These proteins are amphiphilic, meaning they have a hydrophilic surface with an affinity for water, as well as a hydrophobic surface that repels water. Wu knew that nano-sized crystals of cellulose are also amphiphilic, so he figured it was worth checking if they could stop ice crystal growth in ice cream. These cellulose nanocrystals (CNCs) are extracted from the plant cell walls of agricultural and forestry byproducts, so they are inexpensive, abundant and renewable.
In a model ice cream — a 25% sucrose solution — the CNCs initially had no effect, says Min Li, a graduate student in Wu’s lab at the University of Tennessee. Though still small, ice crystals were the same size whether CNCs were present or not. But after the model ice cream was stored for a few hours, the researchers found that the CNCs completely shut down the growth of ice crystals, while the crystals continued to enlarge in the untreated model ice cream.
The team’s tests also revealed that the cellulose inhibits ice recrystallization through surface adsorption. CNCs, like antifreeze proteins, appear to stick to the surfaces of ice crystals, preventing them from drawing together and fusing. “This completely contradicted the existing belief that stabilizers inhibit ice recrystallization by increasing viscosity, which was thought to slow diffusion of water molecules,” adds Li, who will present the work at the meeting.
In their latest study, the scientists found that CNCs are more protective than current stabilizers when ice cream is exposed to fluctuating temperatures, such as when the treat is stored in the supermarket and then taken home. The team also discovered the additive can slow the melting of ice crystals, so it could be used to produce slow-melting ice cream. Other labs have shown the stabilizer is nontoxic at the levels needed in food, Wu notes, but the additive would require review by the U.S. Food and Drug Administration.
With further research, CNCs could be used to protect the quality of other foods — such as frozen dough and fish — or perhaps to preserve cells, tissues and organs in biomedicine, Wu says. “At present, a heart must be transplanted within a few hours after being removed from a donor,” he explains. “But this time limit could be eliminated if we could inhibit the growth of ice crystals when the heart is kept at low temperatures.”
The researchers acknowledge support and funding from the U.S. Department of Agriculture’s National Institute of Food and Agriculture, Agriculture and Food Research Initiate (AFRI) project (2019-06761) and Hatch project (1016040).
ACS Spring 2022 will be a vaccination-required and mask-recommended event for all attendees, exhibitors, vendors, and ACS staff who plan to participate in-person in San Diego, CA. For detailed information about the requirement and all ACS safety measures, please visit the ACS website.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org.
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Media Contact
ACS Newsroom
newsroom@acs.org
View larger image

