FOR IMMEDIATE RELEASE
“In Silico Discovery of Small Molecule Modulators Targeting the Achilles' Heel of SARS-CoV-2 Spike Protein”
ACS Central Science
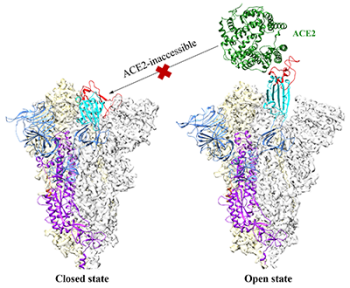
The infamous spike proteins on the surface of SARS-CoV-2 help it bind to and enter human cells. Because of their important role in spreading infection, these spike proteins are one of the main targets for COVID-19 vaccines and treatments. But those remedies gradually lose effectiveness when certain segments of the spike proteins mutate. Now, researchers report in ACS Central Science that they have discovered small molecules that successfully target other segments that mutate less.
Spike proteins change shape when they attack a cell. In their “open” structure, they expose a section known as the receptor-binding domain (RBD) so it can attach to the ACE2 protein on human cells. In the “closed” structure, this RBD segment is tucked inside the spike protein and can’t bind to human cells. Antibodies contained in some COVID-19 therapies or stimulated by vaccines or infection target the RBD domain so it can’t bind to ACE2. However, some emerging variants of the coronavirus contain mutations in the RBD fragment. That means vaccines and antibody therapies designed to target that fragment could become less effective as the virus mutates.
To get around this problem, other, less mutation-prone parts of the spike protein could be targeted instead. One possibility is a pocket in the spike protein that has been dubbed the Achilles’ heel of the virus. When this cranny is occupied by free fatty acids (FFAs) or a few other compounds, the protein remains locked in its closed, harmless configuration. However, those compounds aren’t suitable treatments because they aren’t stable or they bind weakly. So, Jianhui Huang, Niu Huang and colleagues decided to look for other potential treatments that lack these flaws.
Using computer modeling, the team screened a library of small molecules, seeking ones that could slip into this pocket and stick firmly to the spike protein, keeping it in the closed shape. The researchers then used surface plasmon resonance and other techniques to evaluate analogs of these molecules for improved binding and solubility. The resulting compounds, which can bind to spike proteins from the original coronavirus as well as the omicron BA.4 variant, could serve as a starting point for developing broad-spectrum treatments for COVID-19, the team says.
The authors acknowledge support from the Beijing Municipal Science & Technology Commission and Tsinghua University.
###
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org.
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.