FOR IMMEDIATE RELEASE
“Quick Release Anti-Fouling Hydrogels for Solar-Driven Water Purification”
ACS Central Science
Access to clean water is being strained as the human population increases and contamination impacts freshwater sources. Devices currently in development that clean up dirty water using sunlight can only produce up to a few gallons of water each day. But now, researchers in ACS Central Science report how loofah sponges inspired a sunlight-powered porous hydrogel that could potentially purify enough water to satisfy someone’s daily needs — even when it’s cloudy.
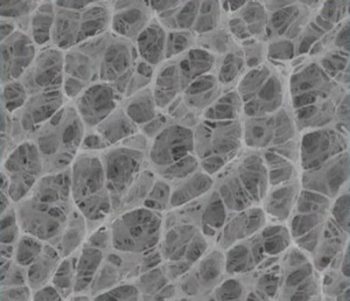
Previously, researchers have suggested that sunlight-driven evaporation could be a low-energy way to purify water, but this approach doesn’t work well when it’s cloudy. One solution could be temperature-responsive hydrogels, specifically poly(N-isopropyl acrylamide) (PNIPAm), that switch from absorbing water at cooler temperatures to repelling it when heated. However, conventional PNIPAm gels can’t generate clean water fast enough to meet people’s daily needs because of their closed-off pores. Conversely, natural loofahs, which many people use to exfoliate in the shower, have large, open and interconnected pores. So, Rodney Priestley, Xiaohui Xu, and colleagues wanted to replicate the loofah’s structure in a PNIPAm-based hydrogel, yielding a material that could rapidly absorb water at room temperature, and rapidly release purified water when heated by the sun’s rays under bright or cloudy conditions.
The researchers used a water and ethylene glycol mixture as a uniquely different polymerization medium to make a PNIPAm hydrogel with an open pore structure, similar to a natural loofah. Then they coated the opaque hydrogel’s inner pores with polydopamine (PDA) and poly(sulfobetaine methacrylate) (PSMBA), and tested this material using an artificial light equivalent to the power of the sun. It absorbed water at room temperature and, when heated by the artificial light, released 70% of its stored water in 10 minutes — a rate four times greater than the one for a previously reported absorber gel. The researchers say that, at this rate, the material has the potential to meet a person’s daily demand. And under lower light conditions, replicating partly cloudy skies, it took 15 to 20 minutes for the material to release a similar amount of stored water.
Finally, the new loofah-like material was tested on samples polluted with organic dyes, heavy metals, oil and microplastics. In all of the tests, the gel made the water substantially cleaner. For example, in two cycles of treatment, water samples with around 40 parts per million (ppm) chromium were absorbed, then released with less than 0.07 ppm chromium — the allowable limit for drinking water. The researchers say the unique hydrogel structure that they created could be useful in additional applications, such as drug delivery, smart sensors and chemical separations.
The authors acknowledge funding from a Princeton University Presidential Postdoctoral Fellowship, a National GEM Consortium Fellowship, the U.S. National Science Foundation, the Eric and Wendy Schmidt Transformative Technology Fund at Princeton University, the Project X Fund at Princeton University and the Princeton Catalysis Initiative.
###
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org.
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.