Eating with Your Eyes: The Chemistry of Food Colorings
By Brian Rohrig October 2015

Would you drink black water? Clear Pepsi? How about using pink butter or green ketchup? Believe it or not, these products actually existed, and not that long ago either. But there is a reason these food fads did not last. Consumers prefer that the color of food matches its flavor.
The link between color and taste is logical. Since oranges are orange, we expect orange-colored drinks to be orange-flavored. Red drinks should taste like cherries, and purple drinks should taste like grapes. If a food is multicolored, it could be moldy and should not be eaten, unless you are eating blue cheese—which gets its distinct flavor from mold!
An astonishing amount of the foods we eat is processed. These foods are altered from their natural states to make them safe, say, to remove harmful bacteria, or to make them appealing and to prolong their shelf life. About 70% of the diet of the average U.S. resident is from processed foods. Much of what we eat would not look appealing if it was not colored. Think of food coloring as cosmetics for your food. Without coloring, hot dogs would be gray. Yum!
Natural Food Coloring
To avoid so much processed food, some have advocated using natural food coloring, whenever possible. Natural dyes have been used for centuries to color food. Some of the most common ones are carotenoids, chlorophyll, anthocyanin, and turmeric.
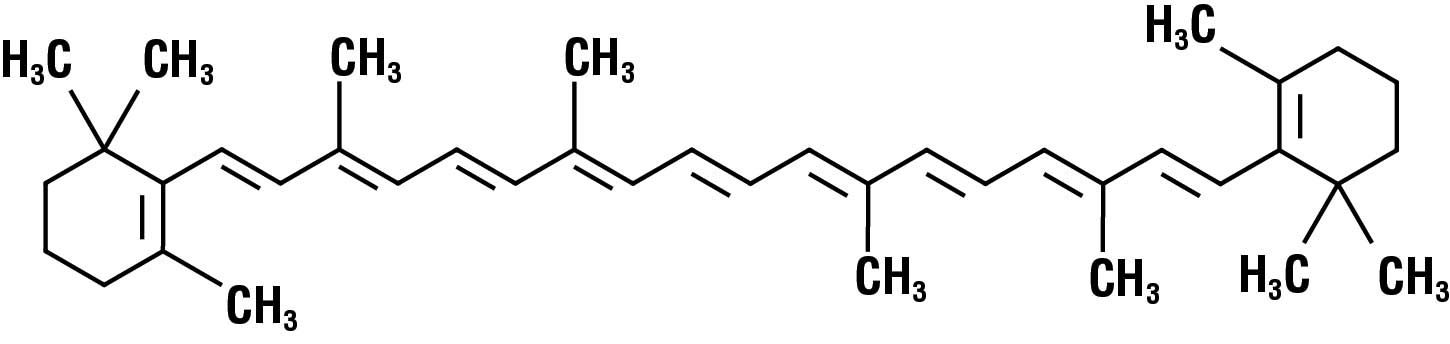
Carotenoids have a deep red, yellow, or orange color. Probably the most common carotenoid is beta-carotene (Fig. 1), which is responsible for the bright orange color of sweet potatoes and pumpkins. Since beta-carotene is soluble in fat, it is a great choice for coloring dairy products, which typically have a high fat content. So beta-carotene is often added to margarine and cheese. And, yes, if you eat too many foods that contain beta-carotene, your skin may turn orange. Fortunately, this condition is harmless.
Chlorophyll is another natural pigment, found in all green plants. This molecule absorbs sunlight and uses its energy to synthesize carbohydrates from carbon dioxide and water. This process is known as photosynthesis and is the basis of life on Earth. Mint- or lime-flavored foods, such as candy and ice cream, are sometimes colored using chlorophyll.
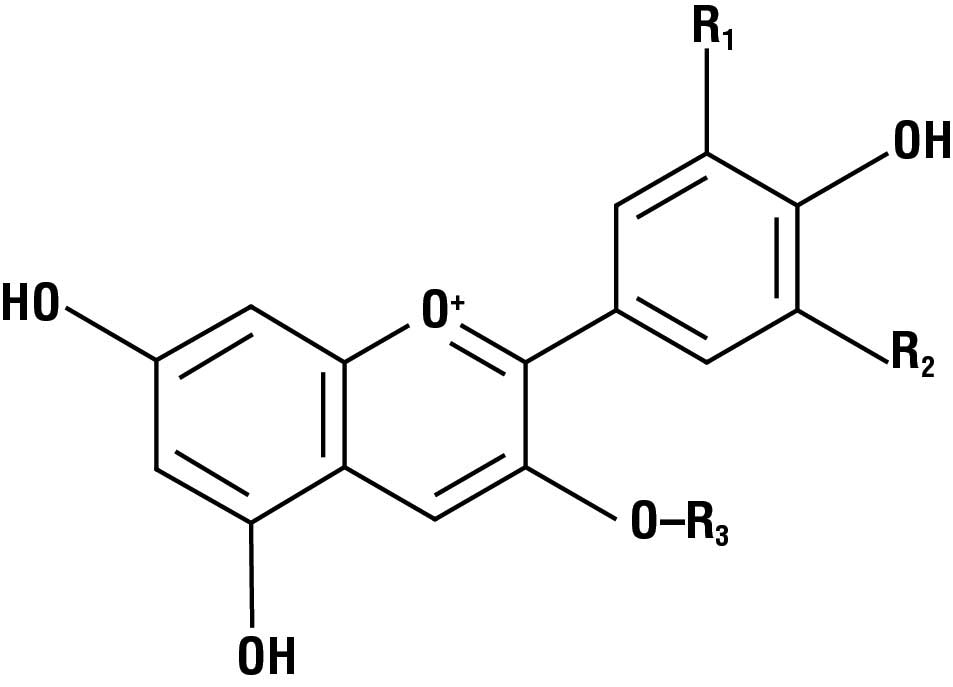
The best natural source for deep purple and blue colors is anthocyanin. Grapes, blueberries, and cranberries owe their rich color to this organic compound. Unlike beta-carotene, anthocyanins—which form a class of similar compounds rather than a single chemical compound—are soluble in water, so they can be used to color water-based products. Blue corn chips, brightly colored soft drinks, and jelly are often dyed with anthocyanins.
More than 500 different anthocyanins have been isolated from plants. They are all based on a single basic core structure, the flavylium ion (Fig. 2). This ion contains three six-carbon rings, as well as many hydroxyl (–OH) groups that make the molecule polar (it has partially negative and partially positive charges) and water-soluble.
Another natural food additive you have probably consumed is turmeric, which is added to mustard to impart a deep yellow color. Turmeric is obtained from the underground stem of a plant that grows in India, and it is commonly used as a spice in Indian food. Many U.S. food companies are using turmeric and other natural spices to color their products. Turmeric is also a great acid/base indicator. If you add a basic substance to mustard, it will turn red.
Bugs, anyone?
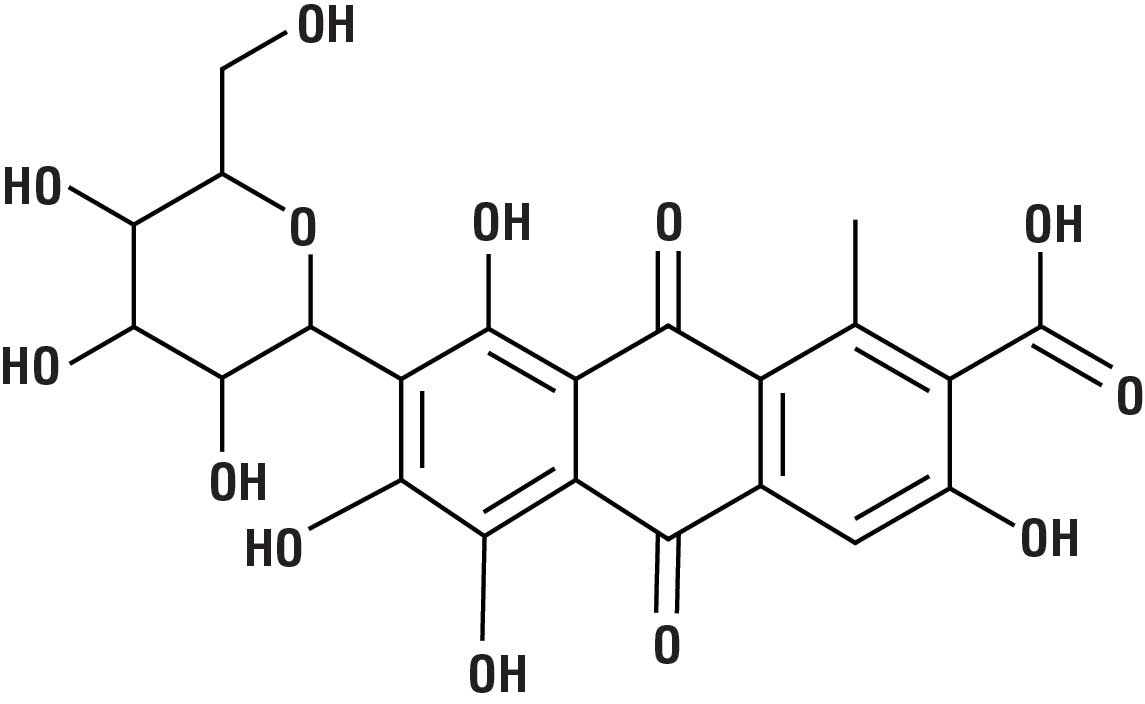
The next time you enjoy strawberry-flavored yogurt or cranberry juice, you may be eating bugs! But don’t worry. These insects did not contaminate your food by accident. An extract from a type of insect, known as the cochineal, was deliberately added by the food manufacturer.
For centuries, the Aztecs used these insects to dye fabrics a deep-red color. If you crush up 70,000 of these bugs, you can extract a pound of a deep-red dye, called carminic acid (C22H20O13 ) (Fig. 3). This dye is safe to ingest, so it found its way into a variety of food and cosmetic products that required a red color. However, the thought of eating bugs is unappealing to some people. Starbucks formerly used cochineal dye in its strawberry-flavored products, but it has since removed this additive in response to customer complaints.
To find out if your food contains bugs, look for carmine, carminic acid, cochineal, or Natural Red 4 on the ingredient label. While these substances are typically considered safe, in rare instances people can have a severe allergic reaction to them, leading to a life-threatening condition called anaphylactic shock.
Why go artificial?
Why bother with artificial, or synthetic, food colorings? Aren’t there enough natural colors to go around? A big reason to go artificial is cost. Synthetic dyes can be mass-produced at a fraction of the cost of gathering and processing the materials used to make natural colorings.
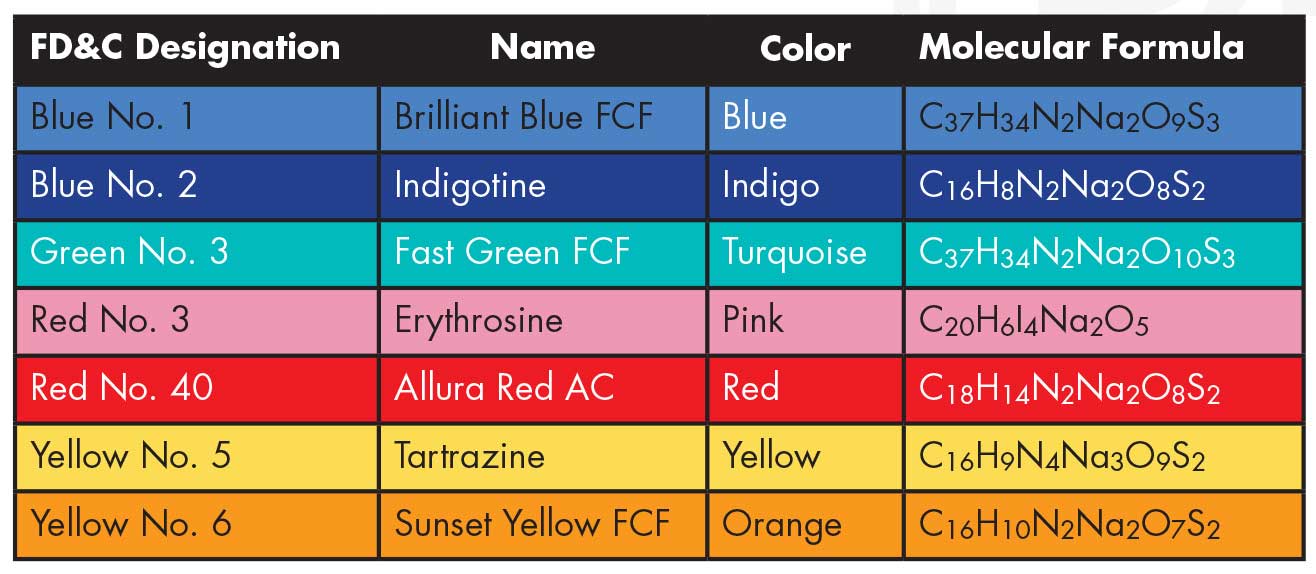
Another reason is shelf life. Artificial dyes might be longer-lasting than natural ones of the same color. Also, although nature produces an impressive hue of colors, those suitable for use as a food dye are limited. But there is no limit to the variety of colors that can be artificially produced in a lab. Considering the thousands of different substances that color our food, it may come as a surprise to discover that the U.S. Food and Drug Administration granted approval to just seven synthetic food colorings for widespread use in food. These food colorings are summarized in Table 1.
Artificial food colorings were originally manufactured from coal tar, which comes from coal. Early critics of artificial food colorings were quick to point this out. Today, most synthetic food dyes are derived from petroleum, or crude oil. Some critics will argue that eating oil is no better than eating coal. But the final products are rigorously tested to make sure they contain no traces of the original petroleum. One dye that does not have a petroleum base is Blue No. 2, or indigotine, which is a synthetic version of the plant-based indigo dye, used to color blue jeans.
How to color food
What makes a good food coloring? First, when added to water, it must dissolve. If the dye is not soluble in water, it does not mix evenly. When a typical solute, such as salt or sugar, is added to water, it dissolves, meaning it is broken down into individual ions or molecules. For instance, individual molecules of sugar (C12H22O11) are held together by relatively weak intermolecular forces. So when sugar dissolves in water, the attractive forces between the individual molecules are overcome, and these molecules are released into solution.
Food-coloring molecules are usually ionic solids, that is, they contain positive and negative ions, which are held together by ionic bonds. When one of these solids dissolves in water, the ions that form the solid are released into the solution, where they become associated with the polar water molecules, which have partially negative and partially positive charges.
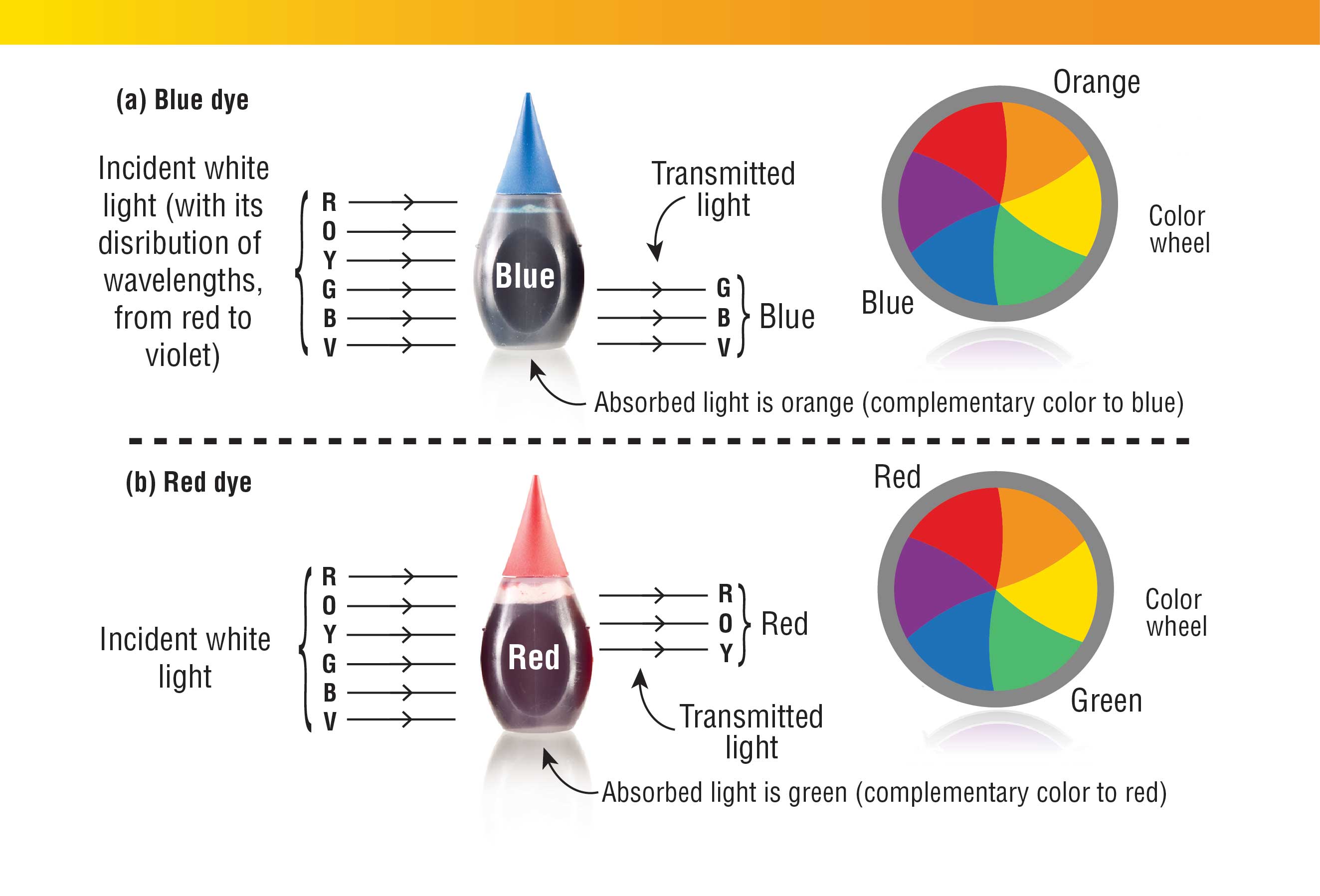
Another important property of food coloring is that when it is dissolved in water, the color remains. The reason this happens is that food-coloring molecules absorb some wavelengths of light and let others pass through, resulting in the color we see (Fig. 4). But why wouldn’t sugar or salt absorb portions of the visible light and scatter the rest of it, like food-coloring molecules do? Absorption of light is caused by bringing an electron in a molecule, atom, or ion to a higher energy level. Sugar molecules or the ions in salt require a large amount of energy to do that, so they do not absorb visible light but only light of shorter wavelength—typically ultraviolet light.
Instead, food-coloring molecules typically contain long swaths of alternating single and double bonds (Figs. 1–3) that allow electrons in these molecules to be excited at relatively low energy. The energy required for an electron to jump from that excited state to the ground state corresponds to the energy of visible light, which is why food-coloring molecules can absorb light from the visible spectrum.
What does the future hold?
It is tempting to think that natural products are healthier than artificial ones. But that is not always the case. Cochineal extract is not the only natural dye that can pose a health risk. Serious allergic reactions have also been reported with annatto and saffron—yellow food colorings derived from natural products.
So what will the food of the future look like? Some advocacy groups, such as the Center for Science in the Public Interest, seek to ban all food coloring, because of limited evidence showing that food coloring encourages children to eat junk food. Others envision a different future. One company has already manufactured an edible spray paint called Food Finish, which can be applied to any food. It comes in red, blue, gold, and silver colors.
Eating involves more than just taste. It is a full sensory experience. Both food scientists and chefs will tell you that the smell, sound, feel, and, yes, the sight of your food are just as important as taste to fully appreciate what you eat. That Slurpee would not taste the same if it did not dye your tongue an electric blue. You really can’t help watching what you eat.
Selected references
McKone, H. T. The Unadulterated History of Food Dyes. ChemMatters, Dec 1999, pp 6–7.
U.S. Food and Drug Administration. Overview of Food Ingredients, Additives and Colors. Nov 2004; revised April 2010: http://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm094211.htm#qa [accessed July 2015].
Fiegl, A. Scientists Make Red Food Dye from Potatoes, Not Bugs. National Geographic, Sept 19, 2013: http://news.nationalgeographic.com/news/2013/09/130919-cochineal-carmine-red-dye-purple-sweet-potato-food-science/ [accessed July 2015].
Borrell, B. Where Does Blue Food Dye Come From? Scientific American, Jan 30, 2009: http://www.scientificamerican.com/article/where-does-blue-food-dye/ [accessed July 2015].
Brian Rohrig is a science writer who lives in Columbus, Ohio. His most recent ChemMatters article, “Smartphones, Smart Chemistry,” appeared in the April/May 2015 issue.
Try this Activity!
Can the Caramel Color of Soda Be Artificially Produced?

The caramel coloring of most commercially manufactured colas is derived naturally from caramelized sugar. Suppose for a moment that you are the chemist who works for a bottling plant. You are in charge of formulating the color for the latest batch of carbonated beverages. Unfortunately, the shipment of natural caramel coloring that you were expecting did not arrive, so you have to make the caramel coloring artificially. Can it be done?
Materials
- Red, blue, and yellow food coloring
- Clear plastic cups
- Eyedroppers
- Sample of commercial cola
- Water
Procedure
- Prepare 3 cups of colored water using the food coloring.
- Pour a sample of the cola in a separate cup. This sample will remain untouched, and will serve as the control you are trying to replicate.
- Using eyedroppers, add colored water from the 3 cups to the single empty cup in an attempt to replicate the color of the cola.
Results
Were you successful? What strategies did you use? Why do you think artificial coloring is typically not used in carbonated beverages?
—Brian Rohrig