Smartphones: Smart Chemistry
April/May 2015
By Brian Rohrig

Could you last a day without your cell phone? As many as 84% of U.S. residents could not, according to a recent poll conducted by Time magazine. It is hard to believe that 20 years ago, hardly anyone even owned a cell phone. And now the cell phone has morphed into something bigger and better—the smartphone. Worldwide, more than one billion smartphones were purchased last year. If you own a smartphone, you are probably aware that in a year or two, it will be practically obsolete, because the smartphone just keeps getting smarter.
In the 1950s, you would have needed a whole bank of computers on an entire floor of an office building to do what you are able to do with a single smartphone today. Even a low-end smartphone has more computing power than the computer system the National Aeronautics and Space Administration (NASA) used to put a man on the moon. Amazingly, you can surf the Internet, listen to music, and text your friends with something that fits in the palm of your hand. None of this would be possible without chemistry, and every time you use your smartphone, you are putting chemistry into action.
Smartphone chemistry
If you are wondering what chemistry has to do with smartphones, just look at the periodic table. Of the 83 stable (nonradioactive) elements, at least 70 of them can be found in smartphones! That’s 84% of all of the stable elements.
Metals are what make smartphones so “smart.” An average smartphone may contain up to 62 different types of metals. One rather obscure group of metals—the rare-earth metals—plays a vital role. These rare-earth metals include scandium and yttrium, as well as elements 57–71. Elements 57–71 are known as the lanthanides, because they begin with the element lanthanum. The lanthanides often appear as the first of two free-floating rows located at the bottom of the periodic table. Scandium and yttrium are included in the rare-earth metals because their chemical properties are similar to those of the lanthanides.
A single iPhone contains eight different rare-earth metals. If you examine several varieties of smartphones, you can find 16 of the 17 rare earth metals. The only one you will not find is promethium, which is radioactive.
Many of the vivid red, blue, and green colors you see on your screen are due to rare-earth metals, which are also used in the phone circuitry and in the speakers. Also, your phone would not be able to vibrate without neodymium and dysprosium.
Rare-earth metals are not only used in smartphones but in many other high-tech devices, too. They are found in televisions, computers, lasers, missiles, camera lenses, fluorescent light bulbs, and catalytic convertors. Rare-earth elements are so important in the electronics, communications, and defense industries that the U.S. Department of Energy dubbed them the “technology metals.”
Smartphone’s display
When shopping for a smartphone, the single most important feature that people look for is the display. The screen allows you to see the phone’s display. If you have ever dropped your phone without damaging the screen, you were probably relieved. Smartphone screens are designed to be extremely tough.
This toughness is actually the result of a serendipitous accident. In 1952, a chemist at Corning Glass Works was trying to heat a sample of glass to 600 °C in a furnace when, unbeknownst to him, a faulty thermostat caused it to be heated to 900 °C. Upon opening the door, he was glad—and surprised—to find that his glass sample was not a melted pile of goo and that it had not ruined the furnace. When he took it out with tongs, he dropped it on the floor (another accident). But instead of breaking, it bounced!
Thus was born the world’s first synthetic glass-ceramic, a material that shares many properties with both glass and ceramic. Glass is an amorphous solid, because it lacks a crystalline structure (Fig. 1(a)). The molecules are not in any kind of order but are arranged more like a liquid, yet they are frozen in place. Because glass does not contain planes of atoms that can slip past each other, there is no way to relieve stress. Excessive stress forms a crack, and molecules on the surface of the crack become separated. As the crack grows, the intensity of the stress increases, more bonds break, and the crack widens until the glass breaks.
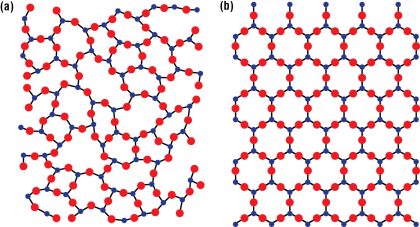
Ceramics, on the other hand, tend to be crystalline (Fig. 1(b)), and they are often characterized by ionic bonds between positive and negative ions—even though they can also contain covalent bonds. When they form crystals, the strong force of attraction between ions of opposite charges in the planes of ions makes it difficult for one plane to slip past another. Ceramics are therefore brittle. They resist compression, but they can break when they are bent.
The combination of glass and ceramic forms a material that is tougher and stronger than each of the materials by themselves. A glass-ceramic is formed by overheating the glass, so a portion of its structure is transformed into a fine-grained crystalline material. Glass-ceramics are at least 50% crystalline, and in some cases, they are more than 95% crystalline.
This amazing glass-ceramic material is so resistant to heat that it has been used in the nose cones of supersonic-guided missiles used by the military. As a result of the success of glass-ceramic materials, the Corning Glass Works Company undertook a large research effort to find ways to make ordinary transparent glass as strong as glass-ceramic products. By 1962, Corning had developed a very strong type of chemically strengthened glass, unlike anything ever seen before. This super-strong glass would eventually make its way to nearly every smartphone screen. It is so strong it goes by the name, Gorilla Glass. Laboratory tests have shown that Gorilla Glass can withstand 100,000 pounds of pressure per square inch!
Gorilla Glass is composed of an oxide of silicon and aluminum—also called aluminosilicate glass—along with sodium ions (Fig. 2).
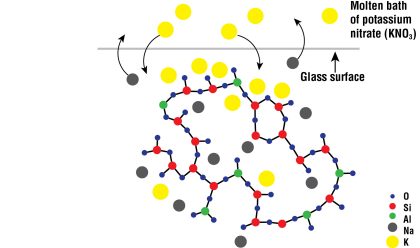
But Gorilla Glass gains its tremendous strength through one final step, in which the glass is chemically strengthened. The glass is put into a molten bath of potassium salt, usually potassium nitrate, at 300 °C. Because the potassium ions are more reactive than sodium ions, they displace them. Potassium atoms are bigger than sodium atoms, and the same holds true for ions—potassium ions are much larger than sodium ions. Therefore, these potassium ions take up more space in the glass than do sodium ions.
Cramming larger ions into the spaces formerly occupied by smaller ions results in a compressing of the glass. Consider this analogy to visualize the process: The world record for the most people crammed into a Volkswagen Beetle, which is a very little car, is 25. These were most likely small people. Now imagine replacing these 25 small people with 25 National Football League linebackers, each weighing in at 350 pounds. To squeeze such large men into such a small space would require a fair amount of compression. Compression will always try to make things smaller.
In the same way, as the larger potassium ions push against each other, the glass is compressed. Compressed glass is very strong. As a result of this compression, a lot of elastic potential energy is stored in the glass, much like the elastic potential energy that you might find in a compressed spring.
What’s behind a touchscreen?
As every smartphone user knows, the screen on a smartphone is far more than just a tough piece of glass. It is a screen that responds to your touch—aptly named a touchscreen—giving you a personal connection to your phone.
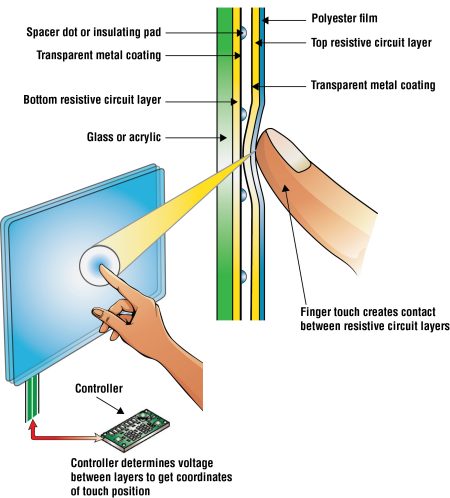
There are two basic categories of touchscreens. The first category of touchscreens, called resistive touchscreens, can be touched with any type of material and they will still work. A pencil works just as well as a finger. You can activate the screen even if wearing gloves. Resistive touchscreens are found in an automated teller machine (ATM) and at checkout counters in stores, where you sign your name for a credit purchase on the display screen.
Resistive touchscreens are composed of two thin layers of conductive material under the surface (Fig. 3). When you press down a resistive touchscreen, it physically indents, causing the two layers to touch, completing the circuit and changing the electrical current at the point of contact. The software recognizes a change in the current at these coordinates and carries out the action that corresponds with that spot. Resistive touchscreens are also known as pressure-sensitive screens. Only one button at a time can be pressed. If two or more buttons are pressed at once, the screen does not respond.
Smartphones use the second basic category of touchscreens, called capacitive touchscreens (Fig. 4), which are electrical in nature. A capacitor is any device that stores electricity.
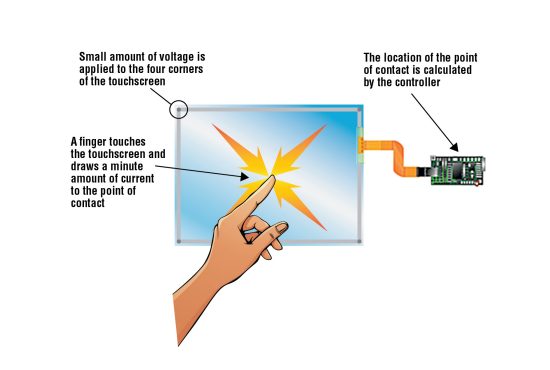
Glass, being an insulator, does not conduct electricity. Even though glass contains ions, they are locked into place, stopping electricity from flowing through. So, the glass screen must be coated with a thin transparent layer of a conductive substance, usually indium tin oxide, which is laid out in crisscrossing thin strips to form a grid pattern.
This conductive grid acts as a capacitor, storing very small electrical charges. When you touch the screen, a tiny bit of this stored electrical charge enters your finger—not enough for you to feel but enough for the screen to detect. As this electrical charge leaves the screen and enters your finger, the screen registers a voltage drop, the location of which is processed by the software, which orders the resulting action.
This tiny bit of electrical current enters your finger because your skin is an electrical conductor—primarily due to the combination of salt and moisture on your fingertips, creating an ionic solution. Your body actually becomes part of the circuit, as a tiny bit of electricity flows through you every time you use the touchscreen on your phone.
Smartphone technology is evolving at a dizzying pace. You can now use your smartphone to check your blood sugar, adjust your home’s thermostat, and start your car. Twenty years ago, no one envisioned that people would someday take more pictures with their cell phones than with their stand-alone cameras. It is anyone’s guess what will come next. Thanks to the intersection of chemistry and innovation, the possibilities are limitless.
Selected references
Gardiner, B. Glass Works: How Corning Created the Ultrathin, Ultrastrong Material of the Future. Wired, Sept 24, 2012: http://www.wired.com/2012/09/ff-corning-gorilla-glass/all/ [accessed Dec 2014].
Ask an Engineer. How Do Touch-Sensitive Screens Work? Massachusetts Institute of Technology, June 7, 2011: http://engineering.mit.edu/ask/how-do-touch-sensitive-screens-work [accessed Dec 2014].
Brian Rohrig is a science writer who lives in Columbus, Ohio. His most recent ChemMatters article, “Air Travel: Separating Fact from Fiction,” appeared in the February/March 2015 issue.
Also in the April/May 2015 Issue....
What should you do with your cell phone when it dies? Recycling it offers both economic and environmental benefits.


