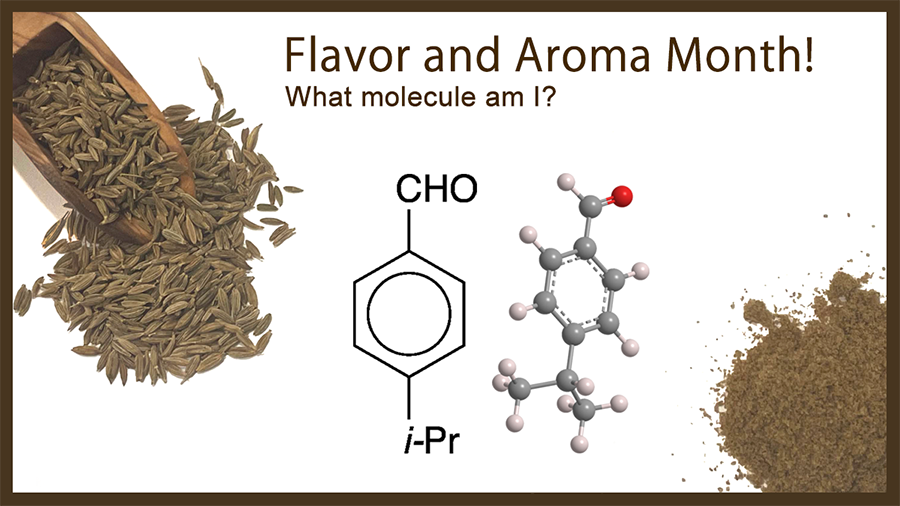
What molecule am I?
November is flavor and aroma month!
Cuminaldehyde, an aromatic aldehyde, gets its name from one of its main natural sources: the cumin plant (Cuminum cyminum) that grows in countries from Turkey across to India. In addition to cumin, cuminaldehyde is found in the essential oils of eucalyptus, myrrh, and cassia.
In small amounts, cuminaldehyde has a pleasant, herbal aroma; however, it also has an acrid, burning taste. As the hazard information table shows, direct exposure can have some unpleasant consequences.
Cumin has been used as a flavoring agent for centuries; cuminaldehyde has been in the chemical literature since at least the 1880s. In volume 2 (1880) of the Journal of the American Chemical Society (then called the American Chemical Journal), an abstract of an article about derivatives of stilbene (1,2-diphenylethylene) describes a reaction between “cumic aldehyde” and phenylacetic acid to form isopropylstilbene.
Almost 70 years later, Nathan N. Crounse at the Hilton-Davis Chemical Co. (Cincinnati) reported a synthesis of cuminaldehyde that uses the high-pressure Gattermann–Koch reaction to formylate cumene (isopropylbenzene) with carbon monoxide. The aldehyde can also be prepared by the reduction of p-isopropylbenzoyl chloride.
Today, most commercial cuminaldehyde is synthetic. It is sold as an ingredient for flavoring agents, perfumes, and other aroma-containing products. It also has several purported medicinal uses (e.g., against pain, inflammation, nausea, and skin conditions), but the effects are largely anecdotal. A derivative, cuminaldehyde thiosemicarbazone1, has been studied for activity against the hepatitis C virus and colon cancer.
A final note: Cumin is the main flavor ingredient in taco sauce. The next time you go out for Taco Tuesday, you have cuminaldehyde to thank.
1. CAS Reg. No. 3811-20-9.
Cuminaldehyde hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 4 | H227—Combustible liquid | |
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal , category 4 | H312—Harmful in contact with skin | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
*Compilation of selected safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Cuminaldehyde fast facts
| CAS Reg. No. | 122-03-2 |
| SciFinder nomenclature | Benzaldehyde, 4-(1-methylethyl)- |
| Empirical formula | C10H12O |
| Molar mass | 148.20 g/mol |
| Appearance | Colorless to yellow liquid |
| Boiling point | 235–236 °C |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

