What molecule am I?
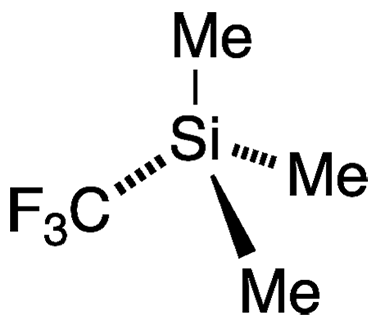
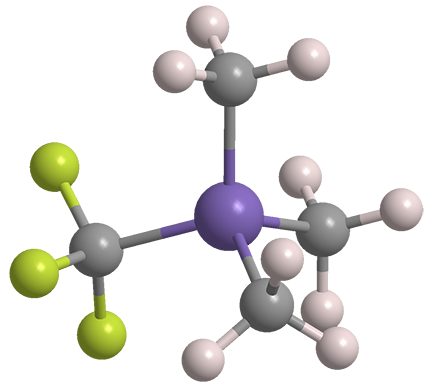
Trifluoromethyltrimethylsilane (TMSCF3) is a fluorinated derivative of tetramethylsilane (TMS), the Molecule of the Week for March 29, 2021. Like TMS, TMSCF3 is a volatile liquid; but the fluorinated compound is considerably more reactive and hazardous.
TMSCF3 is not synthesized from TMS; rather, it is formed by coupling molecules containing trifluoromethyl and trimethylsilyl groups. The initial synthesis, by Ingo Ruppert*, Klaus Schlich, and Wolfgang Volbach at the University of Bonn (Germany) in 1984, involved the reaction of bromotrifluoromethane (CBrF3) and trimethylsilyl chloride (TMSCl) in the presence of an amine–phosphorus catalyst.
Similar methods followed over the next several years, culminating in one reported by G. K. Surya Prakash*, Ramesh Krishnamurti, and George A. Olah*1 at the University of Southern California (Los Angeles) in 1989 that does not require ozone-depleting reagents such as CBrF3. Instead, the researchers treated fluoroform (CHF3) with TMSCl and the base potassium hexamethyldisilazide to obtain TMSCF3 in 80% isolated yield.
Ruppert and Prakash prepared TMSCF3 because they had bigger things in mind. Specifically, they sought to introduce a reagent that could easily add the trifluoromethyl group to a wide range of organic substrates. Now known as the Ruppert–Prakash reagent, TMSCF3 has been used to add CF3 or the difluoromethylene group to aldehydes, ketones, olefins, alkynes, heterocyclic systems, and more.
In 2014, Mang Wang, Qun Liu, and colleagues at Northeast Normal University (Changchun, China) published an excellent review of TMSCF3 chemistry. So much work is being done with TMSCF3 that, even after the conclusion of the review, the authors felt compelled to add an additional section that reported findings that appeared after the original manuscript was submitted for publication.
1. Olah received the Nobel Prize in Chemistry in 1994 for his work on superacids and carbocations.
Trifluoromethyltrimethylsilane hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Flammable liquids, category 2 | H225—Highly flammable liquid and vapor | |
| Substances and mixtures which, in contact with water, emit flammable gases, category 2 | H261—In contact with water, releases flammable gas | |
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Trifluoromethyltrimethylsilane
fast facts
| CAS Reg. No. | 81290-20-2 |
| SciFinder nomenclature | Silane, trimethyl-(trifluoromethyl)- |
| Empirical formula | C4H9F3Si |
| Molar mass | 142.20 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 54–55 ºC |
| Water solubility | Slight decomposition |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

