Visit your local sporting goods shop and you will be met with a dizzying array of choices in athletic performance apparel and clothing for the outdoors. A shirt you think might cost $20 costs $60. What gives?
Welcome to the world of fabric technology. In 2020, people spent $160 billion worldwide on sportswear. The goal is not just to look good, but to feel comfortable, too. Compression garments are designed to massage and support muscles, improving blood flow. Running shoes offer abundant cushioning and traction.
Sweat-wicking fabrics are designed to keep you cool and dry. Do they really work? Are they worth the cost?
If you go for a run on a hot day in a cotton shirt, it will quickly become soaked with sweat. This is not only uncomfortable, but a sweat-saturated shirt can cause no small amount of skin irritation.
While cotton fabrics are soft and comfortable, they are not the best choice for most athletic endeavors. Cotton fabric absorbs and holds on to water like a sponge. Textile engineers (engineers who work with fibers, yarn, and fabric) have coined a term for how much water a fabric retains when wet: the moisture regain value. This value is found by dividing the mass of water in a saturated fabric by the mass of the dry fabric.
For example, if a 100-gram (g) sample of fabric has a mass of 110 g when wet, its moisture regain is 10% (mass of water/mass of dry fabric). The moisture regain values for some common fabrics are as follows:
Cotton » 8.5% | Wool » 16% | Cotton-polyester (50/50) » 4.45% | Nylon » 4% | Polyester » 0.4%
To understand why some fabrics absorb water better than others, let’s take a closer look at water, from a chemist’s perspective.
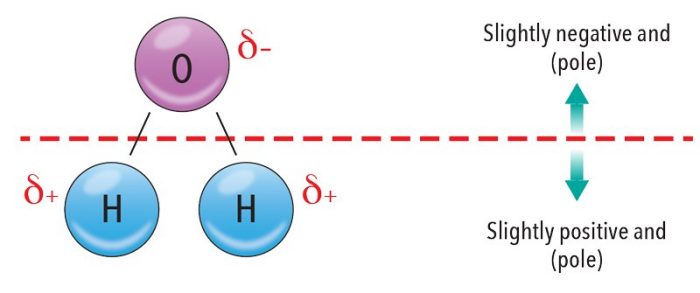
Water molecules are polar—their charges are divided. The word polar is derived from the word “pole;” the charges are distributed as if at the two ends of a stick. Overall, the molecule is neutral, but its negative charges congregate on one side while excess positives remain on the other.
Atoms form bonds by sharing their outer (valence) electrons, but this sharing isn’t always even. In water, oxygen has a greater pull for the shared electrons than hydrogen. Where the shared electrons spend more of their time is controlled by a property known as electronegativity. Electronegativity is a measure of an atom’s ability to attract shared electrons in a chemical bond.
Because the oxygen atom has a greater electronegativity, it draws electrons closer to itself and the oxygen side of the molecule becomes slightly more negative. The electrons are now closer to oxygen, which exposes the positive nuclei of the hydrogen atoms, making the hydrogen side of the molecule slightly positive.
Water doesn’t have full charges, as in ionic compounds, but rather partial negative (δ-) and partial positive (δ+) charges. We use partial charges because even though the electrons spend the majority of their time with the oxygen atom, they still spend some of their time with the hydrogens. Nonpolar molecules share their electrons evenly, or symmetrically, so there is no partial positive or negative side.
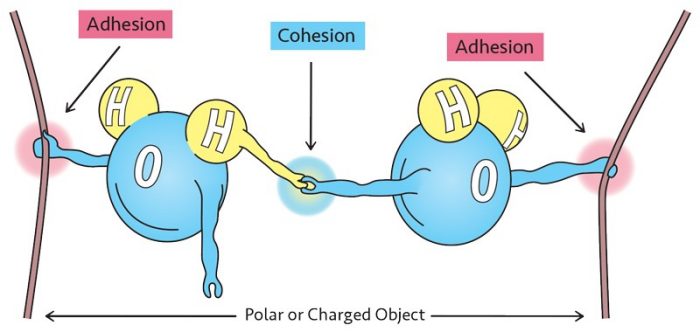
The polarity of water contributes to all sorts of interesting phenomena. Water droplets form because billions and billions of water molecules stick together through an intermolecular force of attraction known as hydrogen bonding. Hydrogen bonding is the attraction between the positive hydrogen side of one water molecule and the negative oxygen side of another water molecule. The attraction of like things is termed cohesion. Because of the strong attraction between molecules due to hydrogen bonding, water is said to be highly cohesive.
Adhesion, in which different things stick together, is more involved. Water droplets that stick to the end of your finger when you dip it in water are held by adhesion. This happens because there are enough polar charges on your skin to attract the water molecule.
One of the most significant phenomenon that depends on the polarity of molecules is the solubility of one type of molecule in another. The polarity of molecules determines what they are attracted to, according to the “like dissolves like” principle. Salt and sugar both dissolve in water. Salt and sugar are both polar. Polar things are attracted to other polar things.
Oil, being nonpolar, will not dissolve in a polar substance. Nonpolar substances, however, will tend to dissolve in other nonpolar substances. Oil will dissolve in olive oil, but not in water.
Another phenomenon based on polarity is how a liquid may spread out on a surface. If a polar liquid is placed on a nonpolar surface, it tends to bead. You may have seen this when water lands on a newly waxed car. The water beads into droplets. When the car is dirty, the water spreads out much more, due to all the polar molecules in the dirt.
One final example of a phenomenon based on these forces is capillary action. Capillary action occurs when the forces of adhesion are stronger than the forces of cohesion. If water molecules have a stronger attraction to the sides of a narrow tube than to one another, they will creep upward.
Capillary action is responsible for a variety of phenomena, from the movement of water in plants to the filling of our tear ducts. Paper towels make use of capillary action, containing tiny channels for water to travel through, greatly increasing their absorbency. Put a strip of paper towel in water and the water wicks up the strip.
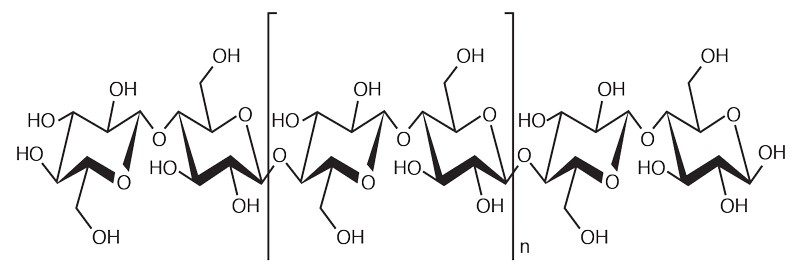
Can you now explain why that cotton shirt becomes so drenched? If you guessed that cotton fibers are polar, you are correct, sort of. Cotton is primarily made of cellulose, a polymer (or macromolecule) made of many glucose molecules strongly linked together, with the basic formula (C6H10O5)n.
Cellulose is a fiber that is found in all plants. But cellulose is not water soluble, otherwise that cotton shirt would dissolve when it got wet.
There are parts of the cellulose that are polar. If you zoomed in to the molecular level, you would notice a bunch of little -OH’s, known as hydroxyl groups, sticking out, like thorns on a rose stem.
Each hydroxyl group is composed of one oxygen atom and one hydrogen atom. Since oxygen is more electronegative than hydrogen, these groups are polar and have a strong attraction for water. As a result, cellulose is strongly attracted to water.
So, if we don’t want to work out in a hydrophilic fabric, do we want to work out in a hydrophobic (lacking an affinity to water) one? If you’ve ever weathered a storm in a plastic raincoat, you soon realized you were just as wet on the inside as on the outside.
This leaves your perspiration with nowhere to go, so it just sits against your skin, which explains why you get that wet and clammy feeling. If you place a drop of water on plastic and it is repelled, demonstrating its hydrophobicity, it is most definitely not sweat wicking.
Wouldn’t it be great if there was a fabric that allowed your perspiration to pass right through, that didn’t keep it against your skin but didn’t absorb it either? That’s exactly what sweat-wicking fabrics are designed to do.
Sweat-wicking fabrics work due to capillary action—the tendency of a liquid to move upward in a narrow tube or space, in apparent defiance of gravity.
The first step in designing a sweat-wicking fabric is to find the right fiber. A fiber is the base material from which clothing is made, and can be natural like cotton or wool, or synthetic like nylon or polyester.
A fiber is a threadlike strand. If a bunch of fibers are twisted together, you have a strand of yarn. When strands of yarn are woven together, you have fabric or cloth. The fabric is then cut and sewn together to make the clothes we wear.

Fibers are the raw material used to make textile items.
They are spun or twisted together to make yarns.

Yarns are made from fibers from either natural or synthetic sources.
They are interlaced, interlooped, or bonded together to make fabrics.
Fabrics are made from yarns.
Different fabric types are produced by different methods of joining the yarns together.
It is important that the fiber is not too hydrophilic, like cotton, which won’t wick away moisture at all. But purely hydrophobic fibers that repel water won’t work either. As the goal is for capillary action to occur, there must be some attraction to water—just not too much.
One popular fabric used in sweat-wicking clothing is polyester. Being petroleum based, polyester is quite hydrophobic, with a moisture regain of only 0.4%. To make it a little less so, it can be chemically treated with a hydrophilic coating. Or the polyester fibers may be interwoven with hydrophilic fibers, forming a blend, which can create an excellent moisture-wicking garment.
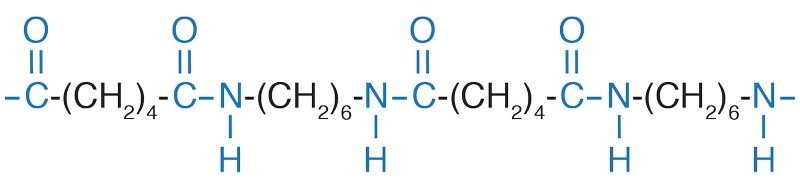
Nylon is a popular choice for sweat-wicking clothing. Nylon is also known as polyamide, because it is composed of repeating units of amides, each containing a carbonyl (C=O) group linked by a single bond to a nitrogen atom. Since amide groups are polar, nylon is hydrophilic—it attracts water.
But nylon is not nearly as hydrophilic as cotton, which contains the highly polar hydroxyl group. An amide group contains nitrogen, which is not nearly as electronegative as the oxygen in the hydroxyl group, so nylon is not as hydrophilic as cotton. But it is just hydrophilic enough.
Spandex, the stretchy fabric commonly found in leggings and bike shorts, has only moderate wicking ability. Spandex is mostly made of polyurethane, but you will never find a garment made of pure spandex—it is usually used in conjunction with nylon or polyester. So, yoga pants not only allow for freedom of movement, they also keep you dry, even during intense workouts.
Some types of sweat-wicking clothing have an inner layer next to the skin that is somewhat hydrophobic and an outer layer that is hydrophilic, creating a push-pull effect. As the hydrophobic layer repels water, moisture is pushed upward into the hydrophilic layer, where it can evaporate.
Wool, especially Merino wool, has excellent moisture-wicking properties. Wool fibers are hydrophilic on the inside but hydrophobic on the outside, due to the presence of lanolin, a waxy material secreted by the sheep’s sebaceous glands, enabling their coats to repel water.
The next step in creating a sweat-wicking fabric requires the most ingenuity: The yarn must be constructed in such a way as to allow capillary action to occur. Most yarns have a circular cross section. When woven together, they fit snugly. There is little room for water to travel between the strands. Their ability to wick moisture is limited.
But if the yarn is designed with spaces between the strands, then moisture can travel through these spaces via capillary action. This amazing little feat of engineering is accomplished by constructing yarns with noncircular cross sections—they may be triangular, cross-shaped, or some other odd shape.
When these strands of yarn are placed together, they do not fit snugly. There is space between them. These little spaces create micropores that are just the right size to facilitate capillary action. Moisture can freely travel through these pores to the outer surface—almost like magic, sweat is wicked away.
How Wicking Yarn Works
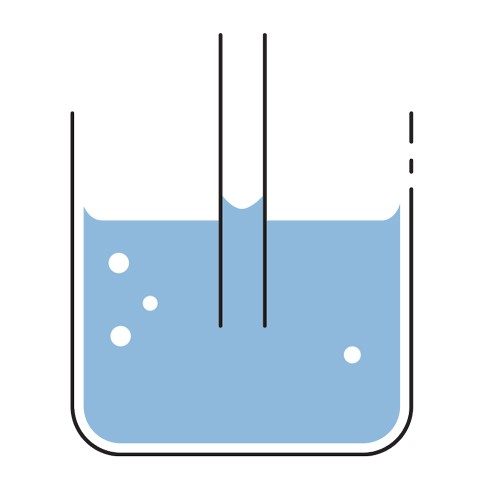
Put a tube in water, the water in the tube is higher due to capillary action.
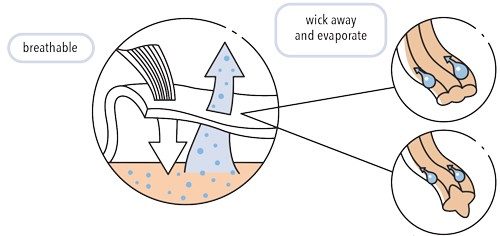
Little tubes in the yarn wick away sweat, which can evaporate in the air.
The magic doesn’t happen, though, without a lot of rigorous testing. Before ever hitting the market, a battery of tests will be performed to make sure a fabric will perform as predicted. These experiments are easy enough to perform yourself—all you need is water and some samples of fabric.
In a vertical wicking test, two strips of cloth are suspended with their ends in water. The fabric in which water travels the fastest will have the greatest wicking ability.
In a horizontal wicking test, a predetermined amount of water is dripped onto the fabric and the horizontal spread of the water is measured. Fabrics with greater wicking ability will show larger spreads in a given amount of time
A transverse wicking test most closely mimics what a moisture-wicking garment is designed to do. Water is absorbed from below the fabric, and its rate of spread through the fabric and along its surface is measured. The faster the spread, the better.
For clothing to keep you cool and dry, sweat must evaporate once it reaches the surface of the garment. Sweating itself does not cool you, it is the evaporation of sweat that cools you. And because evaporation is endothermic, it must absorb energy.
When liquid water vaporizes, the weak cohesive bonds between the water molecules are broken. It takes energy to break a bond. This energy comes from your body, thus cooling you down.
Making choices to make you feel cool and comfortable when you are working out are much easier with the advent of high-tech fabrics that allow moisture to be wicked away. Thanks to a good understanding of fabrics and the nature of water, there are plenty of choices. Looking cool is another matter. You’re still on your own for that!
REFERENCES
Priyalatha, S.; Dhanakodi, D. R. A Multi-Directional Wicking Instrument to Measure Wicking Characteristics of Fabrics Under Dynamic Movements. Journal of the Institution of Engineers (India), September 2018: https://www.researchgate.net/publication/327712897_Wicking_Instrument [accessed August 2022].
Miao, M.; Xin, J. H., Eds. Engineering of High-Performance Textiles. United Kingdom: Elsevier Science, September 2017: https://www.elsevier.com/books/engineering-of-high-performance-textiles/miao/978-0-08-101273-4 [accessed August 2022].
Crow, R.; Dewar, M. Liquid Transport Across Fabric Layers. Defense Research Establishment Ottawa, Report No. 1002, March 1989: https://apps.dtic.mil/dtic/tr/fulltext/u2/a209567.pdf [accessed August 2022].