In 2010, a powerful 7.1 magnitude earthquake hit Haiti. International aid organizations raced there to provide food, medicine, and shelter, but overlooked was the need for light and a way to power emergency cell phones.
While batteries can provide energy when no electric grid is available, lights and cell phones draw a lot of power and thus their batteries must be replaced or recharged regularly—a challenge during a crisis.
Andrea Sreshta and Anna Stork were inspired to develop cheap solar powered lights with built-in phone chargers. Solar lanterns are now distributed by humanitarian groups in disaster areas or war zones, used by hikers in remote areas, and kept in homes in case of power outages.
Solar-powered products harvest energy from the sun and store it in batteries for use at night or on cloudy days. Batteries charged by solar power also move the rover on Mars and support science experi-ments in remote areas.
One research project in Antarctica needed equipment to work through the winter after the scientists departed. Car-sized batteries with attached solar panels store enough energy to run for about two weeks without the sun.
“When the sun comes back, the solar panel starts charging the battery,” says Senior Staff Scientist Pnina Miller. “The equipment turns back on, all by itself. Then we go back in the summertime and retrieve the data,” she said.
While solar power has the potential to power everything from lights to entire households, it is only as good as its storage battery. Batteries can keep things running in extreme environments, or in our daily lives, but they come with challenges and costs.

The Benefits of Batteries
In scientific terms, energy is the ability to do work. Modern life uses energy for transportation, running electronics, powering appliances, lighting, and heating and cooling buildings—and the amount of energy people use grows with each generation.
Unfortunately, about two-thirds of the energy produced worldwide is lost before it reaches customers. Energy gets lost as it is converted to electricity, largely as heat energy. Sometimes energy is poorly used: Lights stay on in empty rooms, outdated equipment doesn’t work efficiently, while other energy is collected but not used.
Batteries can store energy through electrochemistry in which electricity is generated by the movement of electrons from one element to another in a reaction known as oxidation-reduction, or redox for short.
In a redox reaction, one species loses electrons as it undergoes oxidation, while another species gains electrons as it is reduced. An electrochemical cell converts chemical energy into electrical energy or takes electrical energy and converts it into chemical energy.
Every battery is an electrochemical cell or a series of cells. Each cell contains two electrodes: the cathode, where reduction (gaining of electrons) takes place, and the anode, where oxidation (loss of electrons) takes place.
These electrodes are electrical conductors used to make contact with an electrolyte: A sub-stance, usually a liquid, that contains ions. Ions are atoms or molecules with an electrical charge.
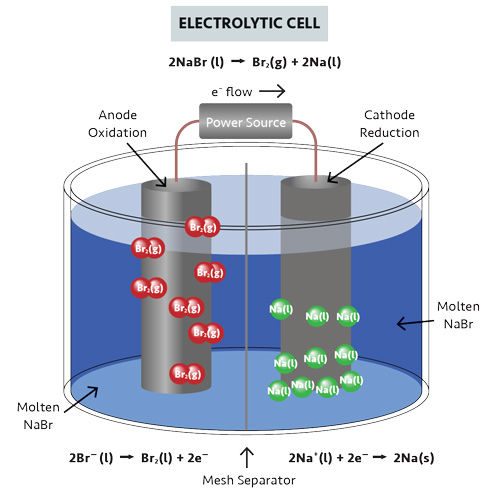
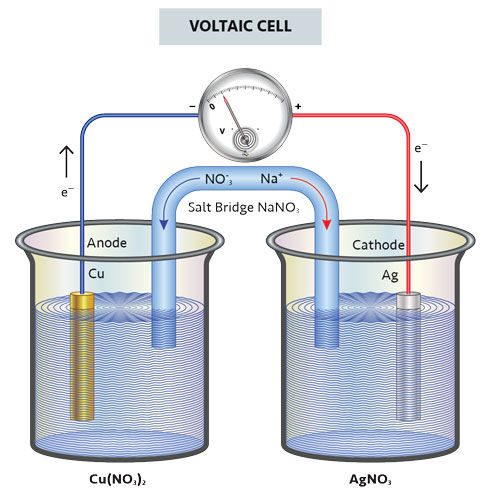
Electrochemical cells that work spontaneously (a reaction occurs by itself) to produce a flow of electrons through a spontaneous redox reaction are voltaic cells, while electrochemical cells that use electrical energy to power a nonspontaneous redox reaction are electrolytic cells.
Rechargeable batteries, like the ones in your cell phone or laptop, act as both a voltaic cell and electrolytic cell. When you use your device but aren’t plugged in, the batteries are discharging spontaneously: a voltaic cell.
Then, when you recharge the battery, they become electrolytic cells, where electricity flows into the system from the power source. This is nonspontaneous and forces the flow of electrons and ions to reverse.
Whether your battery is accepting or releasing energy, nothing happens until it is coupled to an external circuit. The electrons must have a completed pathway to flow through.
Credit for the first usable battery is generally given to Italian physicist Alessandro Volta, who built a simple battery in the early 19th century. His voltaic pile device used alternating zinc and silver discs.
Volta separated the discs with layers of cloth or paper soaked in either sodium hydroxide or salt-water to form the electrolyte. Volta’s pile underwent a spontaneous reaction and, thus, spontaneous electrochemical cells are called voltaic cells.
Around 1834, Michael Faraday experimented with the voltaic pile and derived the quantitative laws of electrochemistry, the study of how electricity relates to chemical reactions. Electricity can be gener-ated by movements of electrons from one element to another.
Advances in materials eventually produced smaller, more powerful batteries, which are now used in all types of portable equipment.
Disposable or Renewable?
Batteries can make energy portable or save it for a rainy day, but batteries run down and stop working over time. Some batteries must be discarded at that point, while others can be recharged.
Rechargeable batteries work the same way as nonrechargeables, except the redox reaction can be reversed using an external energy source. For example, plugging a battery charger into a wall socket provides direct current voltage to recharge the battery.
Rechargeable batteries can be used hundreds of times, delaying the need for new batteries. That means less mining for resources, less energy spent recycling dead batteries, and fewer toxic materials in landfills.
So, why do we have disposable batteries? They’re cheaper, and they can hold a charge for years when not used, making them better for important but seldom-used items such as emergency flashlights.
Disposable lithium-iodine batteries find use in medical implants such as pacemakers and places where replacing or recharging batteries is difficult. These batteries use a lithium anode and a solid complex of iodine (I2) as the cathode. The electrodes are separated by a layer of solid lithium iodide (LiI), which acts as the electrolyte by allowing the diffusion of Li+ ions. The solid electrolyte causes resistance, so only a low current can be drawn, but they are reliable and last a long time.
Rechargeable Lithium-ion Batteries
Among rechargeable options, lithium-ion batteries (LIB) are common for home use. LIBs have an anode made of very pure graphite (the same material found in a pencil), and the cathode is made of a mixed metal oxide containing lithium, oxygen and a transition element like cobalt. Electrons flow between these electrodes via a device, while the lithium ions travel through the separator.
When the battery is discharged, the lithium atoms stored in the graphite anode are oxidized, creating lithium cations and electrons. Electrons travel through the device, while lithium cations move through the electrolyte to the cathode. At the cathode, the electrons reduce the cobalt making the metal oxide negatively charged. This negative charge is balanced by the incoming lithium cations.
When the battery is charged, the flow of electrons is reversed. The cobalt is oxidized, and the electrons are pushed back to the graphite by the external power source. As the metal oxide loses its negative charge and the graphite gains it, the lithium ions are attracted back to the graphite. The charge is then transferred from the graphite to the lithium cations to make neutral lithium atoms.
Although relatively expensive, LIBs perform well in devices like laptops and cell phones, particularly those that drain power quickly.
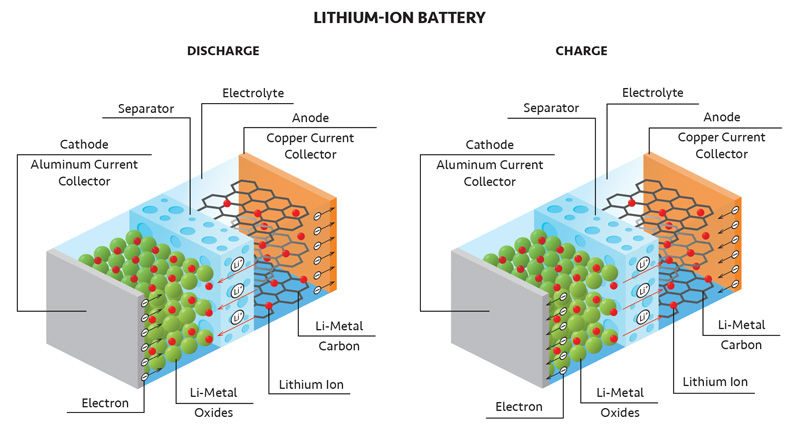
The distinction between discharge and charge in a Lithium-ion battery lies in the direction of electron flow. When discharging the reaction is spontaneous and the electrons move from the lithium within the graphite through the device to the metal oxide. When charging the reaction is reversed by an external power source and electrons move from the metal oxide to the graphite and lithium.
The Environmental Impact of Batteries
In addition, LIBs are often used for storing energy on the electric grid and in electric vehicles (EVs). People who buy EVs generally want to help the environment with reduced carbon emissions and minimize reliance on fossil fuels; unfortunately, EV batteries are another source of pollution.
“The most common materials used in LIBs are lithium, graphite, and metals such as cobalt, nickel, and manganese,” says analytical chemist Sarah Hendrickson, who has worked on green-battery research.
“Lithium mining uses a lot of water—approximately a half a million liters, or 130,000 gallons, of water to extract a ton of lithium.” Mining for battery components has caused toxic chemical leaks, polluted water systems, and poisoned animals and miners.
The damage doesn’t end there. Batteries in landfills can leak dangerous metals and concentrated aqueous solutions of potentially corrosive salts. In some countries, regulations require battery recycling to keep the pollution out of landfills and reduce the need for new materials.
But recycling doesn’t always solve the problem. Most lead recycling is done in economically disadvantaged areas with few pollution controls, so lead is released into the air, soil, and water.
Plus, improper storage and handling of batteries causes hundreds of fires each year in Canada and the United States alone.
Better Batteries
Many governments and private companies are investing in research and commercialization of alternative batteries. Researchers are working to develop batteries that use materials that are less toxic and easier to obtain.
Lithium-sulfur batteries use a lithium anode with a cathode of sulfur and carbon instead of cobalt and nickel, removing some of the metals that cause environmental damage when mined. They have higher-energy density than LIBs, meaning they store more energy in the same amount of material. These batteries degrade quickly, however, as sulfur is lost into the electrolyte.
Sodium-ion batteries use sodium in place of lithium. These are highly efficient, don’t require as much mining, and are potentially cheaper, because sodium is more abundant than lithium. They have a lower-energy density than LIBs, however. They can’t store as much energy in the same amount of material, so it’s hard to make them small and long-lasting.
Researchers are also working on lithium-air batteries that use a solid electrolyte, made from a ceramic polymer material, instead of a liquid electrolyte. This allows the battery to use oxygen from the air and removes the corrosive liquid electrolyte that breaks down over time and leaks.
There are many areas of research as scientists try to build batteries that work better with less environmental impact. It’s challenging to identify the “best battery,” because there are so many different uses for them. The power needs vary from electric-grid energy storage, cell phones, and smoke alarms to implanted medical devices.
Fuel Cells: The Future?
Fuel cells are another big area of research. A regular battery contains all the reactants it needs to produce electricity. A fuel cell, on the other hand, needs a constant external supply of reactants.
While that sounds like a disadvantage, a fuel cell will keep making electricity as long as it has a fuel supply, without the need for recharging—an advantage if the fuel is cheap and plentiful.
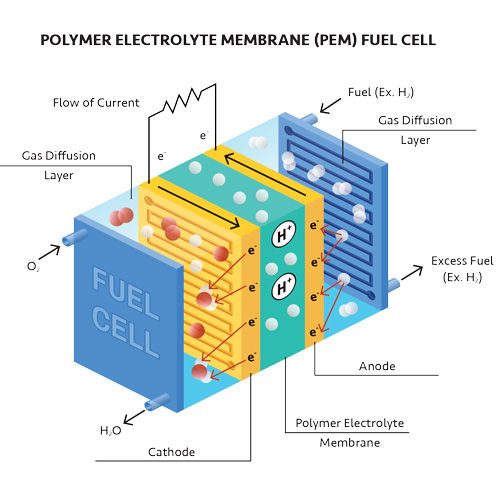
Hydrogen fuel cells use oxygen and hydrogen as fuel. The hydrogen, stored in a fuel tank, is introduced to the anode. Oxygen from the air is introduced to the cathode. The fuel-cell catalyst speeds up the electrochemical reaction.
During this reaction, the hydrogen molecules break apart into two hydrogen ions (H+) and electrons. The hydrogen ions travel through an electrolyte membrane to the cathode.
Meanwhile, the electrons are forced to travel through an external circuit, which provides power to the device or machine. The electrons then join the hydrogen ions at the cathode, where they react with a catalyst and oxygen molecules to form water.
Hydrogen fuel cells do not produce harmful emissions the way burning gasoline does, and in theory, this fuel can be made from renewable sources. Oxygen is in the air. Hydrogen is found in water and plants.
While hydrogen is the most abundant element in the universe it usually exists combined with other molecules, so we can’t easily pluck it from the environment; but improvements in technology have made it, and will continue to make it, easier to produce.
Polymer electrolyte membrane fuel cells use a polymer membrane as the electrolyte and hydrogen as fuel. The direct-methanol fuel cell also has a polymer membrane as the electrolyte, but it uses methanol (a liquid) instead of gaseous hydrogen.
NASA has used alkaline fuel cells as a source of electrical energy and drinking water on space missions. They use an alkaline electrolyte such as potassium hydroxide or an alkaline membrane. Several other fuel-cell types use different materials as the electrolyte. Each has advantages and disadvantages.
Several companies already make electric vehicles using hydrogen fuel cells, but these require a hydrogen-fueling station. At this time, California is the only U.S. state that has hydrogen fuel stations.
Hydrogen fuel-cell cars won’t become common until hydrogen is widely available. That’s a big rea-son why it’s been hard to replace gas-powered vehicles—the gasoline fuel system is already in place.
The Search for Alternatives
The U.S. Department of Energy, national laboratories, universities, and businesses are working on the issues together. Companies spend billions of dollars to research, develop, and produce batteries every year.
But in the end, “there is nothing physical you can buy that does not have an environmental impact,” Hendrickson says. “There is an enormous market for green alternatives to just about every product. There are no real guidelines or requirements for something to be dubbed ‘green.’ One must dig deeper to determine the real cost or benefit, and batteries are no exception.”
Hendrickson contends that over time, “batteries will become cleaner and more powerful, just as the internal combustion engine has.”
In the meantime, we can reduce energy use, use rechargeable batteries, pressure companies to develop better batteries, and try to properly recycle batteries. “Stay curious and keep asking questions,” Hendrickson adds. You might just be one of the next generation of scientists making breakthroughs that keep us safely energized.
REFERENCES
Types of Batteries. PNNL. https://www.pnnl.gov/explainer-articles/types-batteries (accessed 2023-12-31).
Nast, C. The Spiralling Environmental Cost of Our Lithium Battery Addiction. Wired UK. https://www.wired.co.uk/article/lithium-batteries-environment-impact (accessed 2023-12-31).
How Sodium Could Change the Game for Batteries. MIT Technology Review. https://www.technologyreview.com/2023/05/11/1072865/how-sodium-could-change-the-game-for-batteries/ (accessed 2023-12-31).
New design for lithium-air battery could offer much longer driving range compared with the lithium-ion battery. Argonne National Laboratory. https://www.anl.gov/article/new-design-for-lithiumair-battery-could-offer-much-longer-driving-range-compared-with-the-lithiumion (accessed 2023-12-31).
Fuel Cells. Energy.gov. https://www.energy.gov/eere/fuelcells/fuel-cells (accessed 2023-12-31).