Compost: Your Trash, Nature's Treasure!
By Julia R. Barrett October/November 2017

CONSIDER ALL OF THE FOOD YOU’VE DISCARDED THIS WEEK—some apple cores, a few handfuls of vegetable peels, the cookie that you dropped on the floor, or the half sandwich that you meant to eat later. Now think about how much food waste you generate each year. It is a massive amount, especially when combined with the contributions from everyone else in your community. If you live in a mid-sized city such as Madison, Wis., for example, you could stand beside nearly 250,000 fellow citizens and gaze with dismay upon several thousand tons of rotting food waste.
But sending organic waste—a chaotic jumble of yard material and discarded food—to a landfill takes up a lot of space. More troublesome, however, is that when organic waste is buried, it decomposes under oxygen-free, or anaerobic, conditions, producing gases that pollute the atmosphere. The decomposition is driven by microbes that grow in the absence of oxygen. So, what’s the best way to handle the issue of disposing of food waste?
Rotting Food
Organic waste is mostly composed of carbon, hydrogen, oxygen, and nitrogen, with small amounts of phosphorus, sulfur, potassium, and other trace elements. When anaerobic microbes decompose the waste, energy is released and the microbes convert the waste into compounds that support their growth and reproduction. Harmful greenhouse gases, such as methane (CH4) and carbon dioxide (CO2), are generated, as well as organic acids and ammonia (NH3).
Although there are natural sources of methane, human actions account for the majority of its emissions globally. Methane, which is useful for fueling items, such as power plants and kitchen stoves, is a potent greenhouse gas—even more so than CO2Municipal solid waste landfills are the third-largest source of human related methane emissions in the United States, accounting for 15.4% of these emissions in 2015. Some of the methane produced in landfills is recovered and used to generate energy. An alternative to reducing methane emissions is to use a more efficient process called composting, which relies on microbial decomposition of organic matter.
Composting
Composting is the microbial breakdown of organic material into simpler components, which are used to fertilize soil without the use of potentially harmful chemicals. Broadly speaking, organic waste can be composted in two ways: 1) anaerobically, which does not require oxygen; and 2) aerobically, which requires oxygen.
Anaerobic composting is similar to the decomposition of organic waste in a landfill, but it is more controlled and releases fewer pollutants into the atmosphere. First, the waste is ground up, because small chunks break down faster than large ones. Then these chunks are loaded into a closed container, called a digester. There, anaerobic microbes consume the organic material to produce methane and a product that looks like a slurry, called a digestate. The primary use of digestate is as a soil conditioner. This organic material can further break down (aerobically) in soil. Because the digester is a closed system, the methane can be captured, stored, and subsequently used as an energy source—none of the methane is allowed to escape, unlike in a landfill. Done properly, anaerobic composting requires expensive equipment so the methane can be captured.
In contrast, aerobic composting occurs in an open system, and therefore in the presence of oxygen. During this process, bacteria—which are a type of microbes—use oxygen and carbon compounds to fuel their growth. The end result of aerobic composting is carbon dioxide, water vapor, and a dark-brown or black organic material. This material, called compost, is about half the volume of the original material and can be used to enrich soil in farm fields and gardens.
Aerobic composting does not require expensive equipment, unlike anaerobic composting. It involves drying the waste and stirring it to maximize its exposure to oxygen—it is a fairly low-tech process.

Finding the right balance
Deciding which type of composting to use depends on the type of organic waste that is available for the system. Food waste decomposes rapidly and produces a substantial amount of methane and carbon dioxide, so an anaerobic digester is usually the favored option.
An anaerobic digester is relatively easy to use. It requires only waste material, a heat source, and water. The microbes work quickly in a hot environment, and the by-products from one type of microbe can be used as fuel for another. But, as mentioned before, the equipment can be pricey.
In contrast, yard waste, leaves, and weeds decompose slowly and do not generate enough methane and carbon dioxide to justify the expense of an anaerobic composting system, so aerobic composting makes more sense if that’s your main type of waste.
An aerobic system requires a lot of work but is more practical because the feedstock isn’t enclosed in a device—you create a pile of compostable waste in open air. The bacteria in an aerobic-composting process work much like the bacteria in anaerobic composting. The bacteria use carbon containing compounds to release energy via chemical reactions, and they use nitrogen to drive their growth, metabolism, and reproduction via proteins and nucleic acids.
Unlike the anaerobic microbes, however, aerobic microbes have more precise needs and require oxygen. Since aerobic composting is more practical for an individual, a family, or a small group, let’s take a closer look at it.
Aerobic composting
Two types of bacteria—called mesophilic and thermophilic—are usually present in organic waste. Mesophilic bacteria grow best in moderate temperatures, typically between 20 °C (68 °F) and 45 °C (113 °F), while thermophilic bacteria grow best in higher temperatures, usually between 40 °C (104 °F) and 65 °C (150 °F).
At the beginning of the process, mesophilic bacteria predominate. As they break down chemical compounds in waste, they generate heat and the compost pile becomes increasingly warmer. One reaction that takes place during aerobic composting is the oxidation of glucose (C6H12O6), a simple sugar present in plants, as follows:
C6H12O6 + 6 O2 ➞ 6 CO2 + 6 H2O + heat energy
Thermophilic bacteria take over when the temperature of the compost exceeds 40 °C (104 °F). As the reactions progress and the organic materials are consumed, the reactions slow, the thermophilic bacteria die off, and the mesophilic bacteria predominate again. Finally, the waste becomes a stable end product called mature compost.
For this process to happen, the compost pile has to support growth and reproduction of both types of bacteria. In addition to the right temperature range, access to oxygen is necessary for a successful compost. To ensure a good supply of oxygen throughout the organic material and to prevent anaerobic processes from kicking in, the compost pile needs to be turned over and fluffed, which also helps release heat. Up to a certain temperature, the heat generated through the bacterial reaction is beneficial, and at about 55 °C (131°F) it is intense enough to kill disease-causing bacteria.
At a temperature of 63 °C (145 °F), the composting material is hot enough to destroy weed seeds. Beyond that temperature, it is too hot for bacteria to survive, and they either die or become dormant spores that can withstand hostile conditions. High temperatures cause water to evaporate from the waste more quickly, leaving the pile dry so it’s unable to support bacteria growth, and it introduces the risk of spontaneous combustion and fire.
Another important factor is having the right amounts of carbon and nitrogen in the system. In general, biological organisms need about 25 times as much carbon (C) as nitrogen (N). Fruit waste, such as an apple, has a carbon-to-nitrogen ratio of 35:1. In this case, there is much more carbon than nitrogen, and the composting process slows. To speed it up, the high C:N ratio of the apple needs to be offset. For example, coffee grounds, which have a C:N ratio of 20:1, could be added to lower the overall ratio of carbon.
Adding too much nitrogen would be a mistake because it can lead to the production of ammonia and other nitrogenrich products, which would cause the composting material to smell bad. Ideally, the C:N ratio should be between 20:1 and 25:1. Bacterial growth doesn’t stop if the ratio is imprecise; it simply slows down.
With bacterial growth nurtured by appropriate oxygen levels, at the right carbon-to-nitrogen ratio, and within a reasonable temperature range, the organic composting material converts into CO2, water, and mature compost fairly quickly. In summer, warm temperatures encourage bacterial activity, so with frequent turning, compost can be ready in about three months. The mature compost can then be spread over soil, so plants can grow and soil can hold carbon. This capability has an underappreciated role in mitigating climate change. Reducing the emissions of methane, a greenhouse gas, is often considered critical, but it is only one advantage—drawing excess carbon out of the atmosphere is the other. Not to mention reducing the amount of organic material discarded in landfills!
Future of composting
The most common use of food waste in the United States is in municipal composting programs. Cities such as San Francisco, Calif.; Portland, Ore.; and Seattle, Wash., have implemented programs that divert organic waste from landfills. In some cities, such as Seattle, it is against the law to put food scraps in the garbage. Other cities, such as Madison, use anaerobic composting to capture methane and they use it to fuel cars, trucks, and buses.
“We’re taking 13,000 tons of food waste to the landfill every year, and instead of paying to bury it, we use it to produce electricity,” says Bryan Johnson, the recycling director in Madison. Young people, in particular, are conscious of protecting the environment and taking responsibility for the products they use. Says Johnson, “People are becoming more aware that even if it’s out of your home, it’s still your responsibility.”
Selected references
Cornell Composting. Cornell Waste Management Institute, Cornell University: http://compost.css.cornell.edu/ [accessed Aug 2017].
Basic Principles of Composting. What is Composting? Louisiana State University Agricultural Center, Research and Extension: http://www.lsuagcenter.com/~/media/system/d/7/c/d57c9aceee429cdcfda31aa37a053688/pub-2622compost2.pdf [accessed Aug 2017].
Sustainable Management of Food Food Recovery Hierarchy. U.S. Environmental Protection Agency, Feb 19,2017; https://www.epa.gov/sustainablemanagement-food/food-recovery-hierarchy [accessed Aug 2017].
Greenhouse Gas Emissions Overview of Greenhouse Gases. U.S. Environmental Protection Agency, April 14, 2017: https://www3.epa.gov/climatechange/ghgemissions/gases/ch4.html [accessed Aug 2017].
Julia R. Barrett is a science writer who lives in Madison, Wis. This is her first article in ChemMatters.
Also in this Issue...
Open for Discussion: Performance Drinks, Are They Safe?
How do you decide whether to choose water or energy drinks when excercising?
Backyard Composting
COMPOSTING CAN BE DONE ON A SMALL SCALE in a backyard bin. In this case, it is best to stick with aerobic composting of fruit and vegetable scraps, yard waste, and other plant matter. Other waste, such as dog and cat feces, would decompose in the pile, but the pile would not be large enough to generate enough heat to kill disease-causing organisms, particularly worm eggs. Meat scraps—including what may have been in your forgotten half-sandwich— and foods that contain high levels of fats and oils are also not recommended for the compost pile. They are more difficult to break down, and they attract pests.
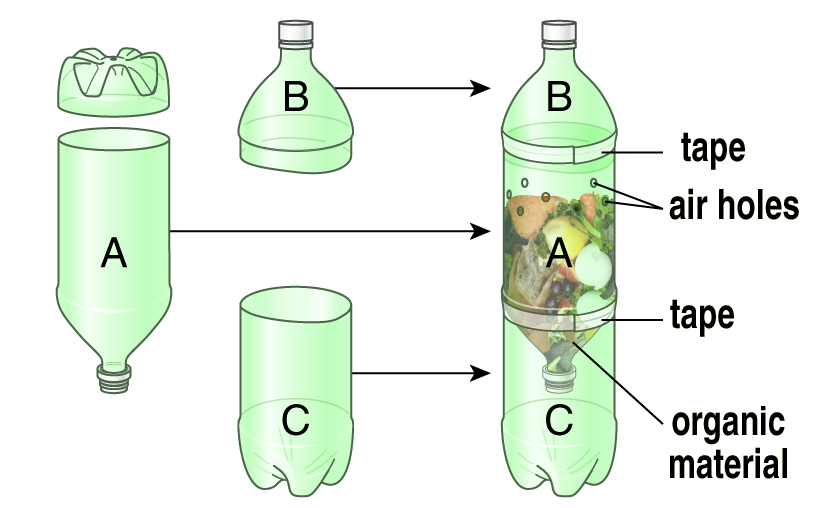
Compost in a Bottle
No yard? No digester? You can still experiment with composting using plastic soda bottles and some plant-based scraps. After assembling your composter following the instructions, set it (or hang it) in a warm spot, such as a sunny window or under a plant light. Check it daily and record observations, such as the appearance of the composting material and any other changes you notice. For example, consider: Are there any odors?, does mold form?, and does it look wet or dry?
As the weeks go by, the scraps will become increasingly less distinguishable from one another, and the overall mass will become smaller. You probably won’t wind up with pure compost, but the material will be different from what you started with.
If you have a lot of soda bottles, conduct more than one experiment—change one factor in each system. What happens if you change the ratio of fruit and vegetable scraps to items with a lot of lignin (for example, newspapers and wood chips)? What happens if you place composters with the same contents in areas with different temperatures? If you add a small amount of partially composted material to fresh material in a new composter, does the decomposition rate change?
You will need:
- three soda bottles
- a nail (or other object for poking holes
- in the bottle)
- a razor blade, or utility knife
- tape, and a few other items
Complete instructions can be found at: http://www.bottlebiology.org/investigations/
decomp_main.html.
Please be cautious when using sharp objects.