Chemistry Takes to the Skies

On Pi Day, March 14, many students compete to see who can recite the most digits of the world’s most famous irrational number. In 2014, students in Austin, Texas, would have had some help with their recitations. An advertising company, AirSign, sent their fleet of small aircraft to emblazon hundreds of digits of pi above the city. The numbers were arranged in a 160-kilometer (100-mile) loop, and each digit was 0.4 km (0.25 mile) high. This “pi in the sky” was a product of the wonderful art of skywriting.
Skywriting wasn’t always so whimsical. The practice dates back to World War I when pilots used it to communicate with one another and create smokescreens to hide their movements from the enemy. It gained popularity in the 1930s as companies took to the skies to send messages to hundreds or even thousands of people at once. Today, skywriting is more of a novelty, representing a unique way to propose marriage or gain a moment of Instagram fame.
But it’s not every day that you can look up at the blue sky and see a message scrawled in puffy white letters or numbers. If you’re a fan of old movies, “The Wizard of Oz” might have been your first introduction to skywriting. In the classic film, the Wicked Witch of the West flies in the air to write the threatening message “SURRENDER DOROTHY” in black smoke with her broomstick. The movie debuted in 1939, when skywriting was at its peak. Of course, the skywriting scene was created with special effects, not actual skywriting. Real skywriting is nearly always white. But the movie did get one thing right—skywriting is created with smoke.
But first, contrails
It’s easy to mistake skywriting for the white streaks in the sky, or contrails, that jet aircraft produce. But there’s a critical chemical difference between the two: Contrails form as water is expelled from the exhaust pipes, while skywriting is made of smoke.
The water responsible for creating contrails isn’t purposely added to the exhaust, but forms as a product of jet fuel combustion. Jet fuel is mostly kerosene, a mix of hydrocarbons, derived from the distillation of crude oil. Hydrocarbons are compounds composed only of carbon and hydrogen.
In chemical terms, the hydrocarbons that make up kerosene are alkanes—hydrocarbons with the general formula CnH2n+2. To determine the number of hydrogens in a specific alkane’s formula, you multiply the number of carbons by 2 and then add 2.
So, if you have a molecule with 10 carbons, it would have 22 hydrogens (10 times 2, plus 2), and its formula would be C10H22. The liquid hydrocarbons in kerosene have anywhere from 10 to 16 carbon atoms, but for simplicity, kerosene is often represented by the formula C12H26.
Here’s where the contrail-forming water enters the picture: Jet engines take in oxygen from the atmosphere, which combines with the fuel and combusts according to the following reaction:
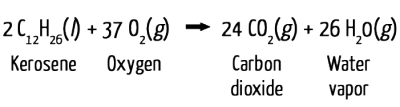
Both of the gaseous products expelled from the exhaust pipe of a jet—CO2 and water vapor—are invisible. When the products hit the cold air, the water vapor condenses and freezes around small particles, known as nucleation sites, which are also present in the exhaust. This produces a visible cloud.
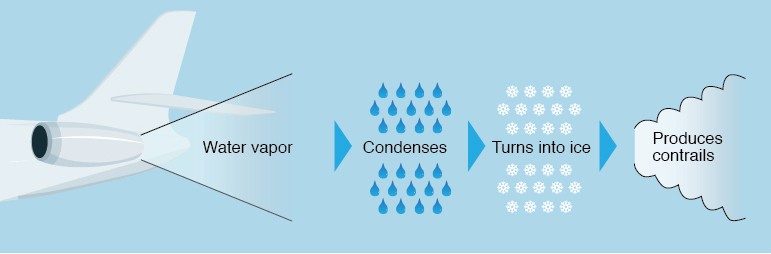
Contrails typically form at an altitude of approximately 9 km (30,000 feet), where low temperatures ensure rapid condensation and freezing.
The relative humidity of the air also plays a big role. After water vapor from the exhaust condenses and freezes to form small crystals, the crystals grow as water vapor from the surrounding air condenses and freezes on the newly formed crystals. Higher levels of relative humidity in the air means more water vapor is available to contribute to the contrail, which makes it last longer.
What is skywriting?
Skywriting typically happens at an altitude of approximately 3 km (10,000 feet) to keep the letters visible for ground-level audiences. But since this altitude is too low for contrails to form reliably, skywriting involves a different process that creates smoke, instead of clouds.
Heating oils with relatively low viscosity, which means they flow easily, can create the
desired effect. Traditionally, skywriters used mineral oil, which you can buy at a drugstore. Canola oil can also be used, and it’s considered more environmentally friendly than mineral oil.
Skywriters carry many gallons of oil in pressurized canisters near the engine. When pilots are ready to make some smoke, they flick a switch, injecting oil into the exhaust manifold, which is the chamber where the gases from the combustion of fuel in the engine collect.
As soon as the mineral oil hits the hot exhaust manifold it instantly boils, because the temperature is much higher than the boiling point of the oil, which is 260 °C (500 °F). From the exhaust manifold, the gases flow through the exhaust pipe and out of the plane.
Like water vapor, gaseous mineral oil is invisible. But as soon as the gaseous oil hits the cold air outside, it condenses, forming plumes of white smoke. If you’ve ever seen oil heated to a high temperature in a frying pan, you may have already seen this type of reaction up close. As the oil boils, its molecules leave the liquid phase. But as the molecules reach the cooler air, the oil condenses and forms smoke.
Smoke is an example of an aerosol: solid or liquid particles suspended in air, and ranging in size from 1 nanometer to 100 micrometers.
Other methods can also yield similar results. One patented method involves the use of titanium(IV) chloride (TiCl4), a liquid that’s clear and volatile, meaning it evaporates easily.
When TiCl4 comes into contact with water in the air, it forms white plumes of titanium(IV) oxide (TiO2), a perfect medium for skywriting. The reaction is as follows:
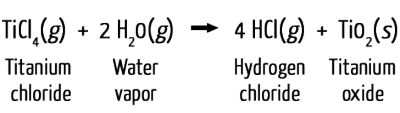
International System of Units (SI) Prefixes
| Number | Power | Prefix | Symbol | |
|---|---|---|---|---|
| One billion | 1,000,000,000 | 109 | giga | G |
| One million | 1,000,000 | 106 | mega | M |
| One thousand | 1,000 | 103 | kilo | k |
| One | 1 | 100 | none | none |
| One-thousanth | 0.001 | 10-3 | milli | m |
| One-millionth | 0.000001 | 10-6 | micro | µ |
| One billionth | 0.000000001 | 10-9 | nano | n |
Buoyant messages—and typos
Knowing how to make smoke is one thing, but using smoke to create a legible message is another matter. Even the most skilled pilot would struggle with skywriting without extensive training. Inexperienced skywriters have been known to make typos in their messages or spell a message backward. If pilots make an error, the best they can do is draw a line through the errant message and start over.
A sky-high typo might feel like a permanent embarrassment to a pilot. But even in calm conditions, the message immediately begins to dissipate, as both the air and particles of smoke are in constant motion. Only their enormous size prevents skywritten letters from quickly becoming unreadable. But within an hour, the words will disappear.
When the letters dissipate, however, the smoke particles remain in the sky. Due to the very small size of its particles, the smoke stays in the air as part of a colloid. Colloids tend to have a cloudy appearance, and are formed when one substance is dispersed in another. Contrails and clouds are examples of colloids.
A novel niche
Since its inception, skywriting has faced stiff competition. Billboard advertising posed the first real threat to skywriting, followed by the creation of TV ads. As a result, the number of skywriters has dwindled—there are only a handful of active skywriters in the world today.
Now, with the Internet, companies can target specific audiences with online ads that don’t require clear skies to be viewed, and they cost very little—as low as a few dollars. By contrast, one simple skywritten message can cost upward of $5,000. At that price, most of us will have to rely on social media to broadcast our messages.
But given skywriting’s rarity, puffy white letters in the sky feel novel again. Some companies have taken advantage of this sentiment for advertising purposes. With hundreds or thousands of people on the ground armed with cellphones, an ad can take to the skies then spread through Instagram or Twitter to thousands more people, for few can resist the magical pull of a message in the sky.
Brian Rohrig is a chemistry teacher who lives in Columbus, Ohio.
References
Layton, J. How Skywriting Works. How Stuff Works: https://science.
howstuffworks.com/transport/flight/modern/skywriting.htm/printable [accessed Dec 2019]
Wilkinson, S. The Sky’s Their Canvas: The Lost Art of Skywriting. HistoryNet: https://www.historynet.com/the-skys-their-canvas.htm [accessed Dec 2019]
LaFrance, A. What Happened to Skywriting? The Atlantic, April 16, 2014: https://www.theatlantic.com/technology/archive/2014/04/what-happened-to-skywriting/360764/ [accessed Dec 2019]
Chemtrails vs. Contrails