What molecule am I?

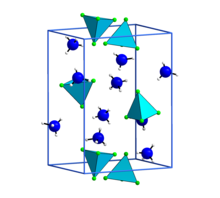
Diammonium tetrafluoroberyllate is one of several salts of the tetrafluoroberyllate anion, in which four fluorine atoms are covalently bonded to a beryllium atom in a tetrahedral arrangement. The anion has also been prepared as salts of alkali metals, alkaline earths, transition metals, and many organic amines, some with varying degrees of hydration.
Also called ammonium beryllium fluoride, the salt is formed by dissolving beryllium hydroxide [Be(OH)2] in an aqueous solution of ammonium hydrogen fluoride [(NH4)HF2]. The aqueous solution is concentrated in an evaporative crystallizer to form the diammonium tetrafluoroberyllate salt. Its crystals are orthorhombic (shown) with a density of 1.71 g/mL. Like all beryllium-containing compounds, it is extremely toxic.
Diammonium tetrafluoroberyllate is used to produce beryllium fluoride (BeF2) glass by heating the salt to ≈1000 °C. BeF2, in turn, can be used to make beryllium metal by reducing it with elemental magnesium at 1300 °C. In the past several years, beryllium has been in short supply; in 2016, L. N. Malyutin and co-workers at Tomsk Polytechnic University (Russia) developed improved methods to purify diammonium tetrafluoroberyllate on its way to making the metal.
Advanced materials manufacturers, such as Materion (Mayfield Heights, OH), use ammonium tetrafluoroberyllate to make their beryllium-containing products. Materion uses the synthetic route described above to make BeF2 and the metal.
In addition to being the precursor to the metal, BeF2 has applications in the nuclear energy industry. Beryllium metal produced from ammonium tetrafluoroberyllate was used to make the mirrors of the James Webb Space Telescope, which is scheduled to be launched later this year.
Ammonium tetrafluoroberyllate hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Sensitization, skin, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 1 | H330—Fatal if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Carcinogenicity, category 1A | H350—May cause cancer by inhalation | |
| Specific target organ toxicity, repeated exposure, category 1 | H372—Causes damage to organs through prolonged or repeated exposure | |
| Hazardous to the aquatic environment, long-term hazard | H411—Toxic to aquatic life with long-lasting effects | |
*Information acquired from online sources, not a specific safety data sheet.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Ammonium tetrafluoroberyllate
fast facts
| CAS Reg. No. | 14874-86-3 |
| SciFinder nomenclature | Beryllate(2–), tetrafluoro-, ammonium (1:2) |
| Empirical formula | H8BeF4N2 |
| Molar mass | 121.08 g/mol |
| Appearance | White crystals |
| Melting point | 280 °C (dec.) |
| Water solubility | 323 g/L |
Over the years, readers have noted that ionic substances are not actually molecules. This is correct, but we use "molecules" in the broadest sense to include them in Molecule of the Week.—Ed.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

