What molecule am I?

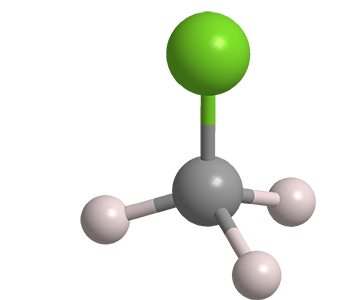
Chloromethane, frequently called methyl chloride, is a colorless, toxic gas. It has been known since the early 19th century.
In 1835, prominent French chemists Jean-Baptiste Dumas of the École Polytechnique and Eugène Péligot of the Institut National Agronomique (both in Paris) teamed up to devise the first synthesis of chloromethane. They heated methanol and sodium chloride in the presence of sulfuric acid to produce the gas. Their synthesis was the forerunner of the primary modern manufacturing method, which uses hydrogen chloride in place of NaCl and H2SO4.
Chloromethane is found sparsely in nature. It is usually produced by the enzyme methyl chloride tranferase, which is present in wood-rotting fungi and salt marsh plants. As of 2020, chloromethane was the only organochlorine compound to have been detected in space, by both the Atacama Large Millimeter/submillimeter Array telescope in Chile and the Rosetta spacecraft.
Chloromethane was once widely used as a refrigerant1, but it has long since been replaced by substances that are less toxic and less harmful to the ozone layer in Earth’s atmosphere. Currently, it is used in industry as a reagent in chemical production, an extractant for oils and resins, a propellant in foam production, and a solvent in rubber manufacture and petroleum refining.
1. As a refrigerant, chloromethane was called Freon-40.
Chloromethane hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Flammable gases, category 1 | H220—Extremely flammable gas | |
| Gases under pressure (liquefied gas), category 3 | H280—Contains gas under pressure; may explode if heated | |
| Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
| Reproductive toxicity, category 2 | H361—Suspected of damaging fertility or the unborn child | |
| Specific target organ** toxicity, repeated exposure, inhalation, category 2 | H373—Causes damage to organs through prolonged or repeated exposure if inhaled | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Explanation of pictograms.
**Central nervous system, liver, and/or urogenital tract.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Chloromethane
fast facts
| CAS Reg. No. | 74-87-3 |
| SciFinder nomenclature | Methane, chloro- |
| Empirical formula | CH3Cl |
| Molar mass | 50.49 g/mol |
| Appearance | Colorless gas |
| Boiling point | –24 °C |
| Water solubility | 5.3 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

