What molecule am I?
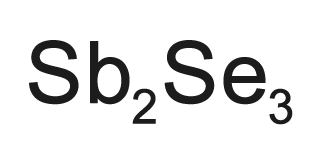
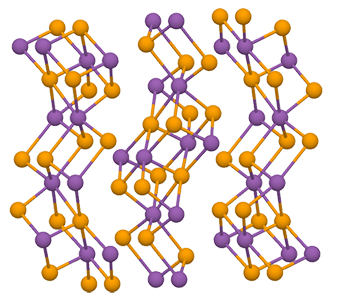
Antimony triselenide (Sb2Se3) is an inorganic compound that occurs in nature as the mineral antimoselite. Its black orthorhombic crystals have a metallic luster. It is primarily found in China, the Czech Republic, and Russia.
French scientist P. Chrétien prepared crude Sb2Se3 from the elements in 1906. In 1924, Ludwig Moser and Kasimir Atynski at the Technical University of Vienna made it in pure form by treating an aqueous solution of potassium antimony tartrate with hydrogen selenide gas.
Historically, Sb2Se3 has been used in pyrotechnics and flares and as an ingredient in paint pigments, glass, matches, and explosives. Most of these uses have been disontinued because of the dangers associated with the compound (see the hazard information table).
But Sb2Se3 is becoming a 21st century player in the field of electronics. As Chuan-Hui Cheng, Weifeng Liu, and colleagues at the Dalian University of Technology, the Chinese Academy of Sciences (Dalian), and Hainan University (all in China) put it, “Antimony selenide (Sb2Se3) is an emerging photovoltaic material.”
In their work to develop high-performance solar cells, the authors note that Sb2Se3 has attractive properties such as “appropriate band gap, high absorption coefficient, single-phase structure at room temperature, inert grain boundaries, earth-abundant constituents, relatively cheap price, and low toxicity”, although that last claim can be called into question.
In any case, the researchers describe their preparation of thin films of crystalline Sb2Se3 as a key component of solar cells. They improved the performance of the films by using a highly aromatic small molecule called NPB1 to induce the crystallization of Sb2Se3 during the annealing process. This refinement increased the photogenerated carrier lifetime of the cell and suppressed interface recombination near the anode.
The Materials Project database contains a profuse amount of information on Sb2Se3, including a rotatable 3-D image of its crystal structure.
1. NPB is N,N’-bis(naphthalen-1-yl)-N,N’-bis(phenyl)benzidine, CAS Registry no. 123847-85-8.
Antimony triselenide hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
| Specific target organ** toxicity, repeated exposure, category 2 | H373—May cause damage to organs through prolonged or repeated exposure | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Explanation of pictograms.
**Central nervous system, liver, and/or digestive tract.
Antimony triselenide
fast facts
| CAS Reg. No. | 1315-05-5 |
| SciFinder nomenclature | Antimony selenide (Sb2Se3) |
| Empirical formula | Sb2Se3 |
| Molar mass | 480.40 g/mol |
| Appearance | Black to gray crystals or powder |
| Melting point | 611 °C |
| Water solubility | Very slight |
MOTW update
Paraquat was the Molecule of the Week for March 17, 2014. It is a widely used but extremely toxic herbicide. It has been banned in the European Union, China, and Brazil; however, its use is still permitted in the United States. It is suspected of causing Parkinson’s disease in farmworkers, as alleged in >100 lawsuits against its producer and distributor; but the US Environmental Protection Agency disputes this claim. This month, despite objections from environmentalists, EPA approved aerial spraying of paraquat under certain conditions.
Over the years, readers have noted that ionic substances are not actually molecules. This is correct, but we use "molecules" in the broadest sense to include them in Molecule of the Week.—Ed.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

