What molecule am I?
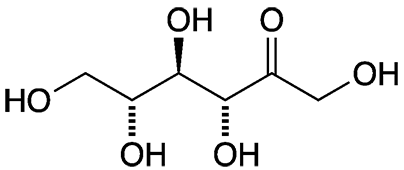
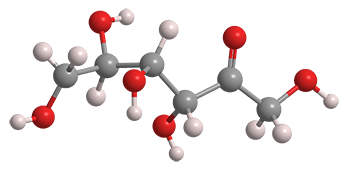
D-Psicose, also widely known as D-allulose, is a natural but rare monosaccharide. It is a ketohexose that has the same empirical formula as common monosaccharides such as glucose and fructose. It is epimeric with fructose at the 3-position. Its enantiomer, L-psicose1, is unknown in nature but has been synthesized.
Known as early as 1915 as D-pseudofructose, German chemists H. Ohle and F. Just determined its structure in 1935 and renamed it D-psicose. In 1942, F. W. Zerban at the New York Sugar Trade Laboratory (New York City) and Louis Sattler at Brooklyn College (NY) isolated it from commercial cane molasses. At the time, it was deemed to be unfermentable and therefore of little practical value.
The physical state of D-psicose is variously reported in the literature as a viscous liquid or a white crystalline solid with a melting point of 58 °C or 109 °C. These discrepancies are probably attributable to the difficulty of crystallizing the compound. At ≈1.0 kg/L, it is extremely soluble in water. (Glucose dissolves at ≈900 g/L, fructose at ≈4 kg/L.)
D-Psicose is 70% as sweet as sucrose (table sugar), but it has only ≈10% of the nutritional energy (calorie) value. As a result, it is under development as a replacement for sucrose and artificial sweeteners. It was originally produced from corn; but this year, food-ingredient startup Bonumose (Charlottesville, VA) and drug-services company Novasep (Lyon, France) teamed up to manufacture D-psicose and fellow low-calorie sugar D-tagatose2 from cellulose, starch, or their derivatives via an enzymatic process.
1. CAS Registry No. 16354-64-6.
2. CAS Registry No. 87-81-0.
D-Psicose hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
D-Psicose fast facts
| CAS Reg. No. | 551-68-8 |
| SciFinder nomenclature | D-Psicose |
| Empirical formula | C6H12O6 |
| Molar mass | 180.16 g/mol |
| Appearance | White crystals or viscous liquid |
| Melting point | See text |
| Water solubility | ≈1.0 kg/L |
MOTW update
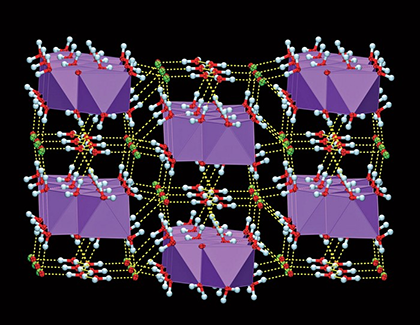
Sodium hypochlorite (NaClO) was the Molecule of the Week for June 30, 2008. It is the active ingredient in “chlorine bleach”. NaClO has been widely used for >200 years; but until now, the crystal structure of its pentahydrate (the normal solid form) had not been established. Tomislav Friščić and co-workers at McGill University (Montreal), working at –100 °C because NaClO•5H2O liquefies at ambient temperature, determined the structure of a single crystal, shown. The structure consists of alternating layers of Na+ and ClO– ions “glued” together by water molecules.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

