What molecule am I?
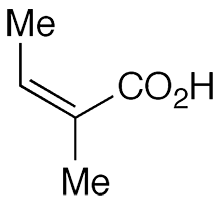
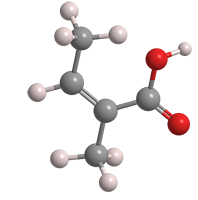
Angelic acid (cis-2-methyl-2-butenoic acid) is an unsaturated aliphatic carboxylic acid that was discovered by Munich pharmacist Ludwig Andreas Buchner in 1842. Buchner isolated it from the carrot-like roots of the herb Angelica archangelica (garden angelica). According to lore, an archangel revealed the herb’s medicinal properties, hence the heavenly name.
Since its discovery, angelic acid and its esters have been found in other plants, such as Roman chamomile (Anthemis nobilis), sabadilla (Schoenocaulon officinale), and lovage (Levisticum officinale). It is often found together with its more thermodynamically stable trans isomer, tiglic acid1.
In 1949, Robert E. Buckles* and Gene V. Mock at Iowa State University (Ames) reported the synthesis of tiglic acid by treating 2-hydroxy-2-methylbutyronitrile2 with sulfuric acid, followed by hydrolysis. They then converted tiglic acid to angelic acid in a three-step process: (1) addition of bromine across the double bond; (2) reaction with potassium hydroxide to form 3-bromoangelic acid3; and (3) reduction with sodium amalgam to produce angelic acid. Six years later, Buckles, Mock, and Louis Locatell, Jr., wrote an extensive review of the chemistry of both isomers.
Angelic and tiglic acids are used primarily as their esters in such products as perfumes and flavorings. The angelic ester petasin4, originally discovered in the common butterbur plant (Petasites hybridus), is a sesquiterpene with potential medical uses.
Despite its name, angelic acid has a spicy—even pungent—odor and a biting, acidic taste. As shown in the hazard information table, it must be handled with care.
1. CAS Reg. No. 80-59-1.
2. CAS Reg. No. 4111-08-4.
3. CAS Reg. No. 35057-99-9.
4. CAS Reg. No. 26577-85-5.
Angelic acid hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Corrosive to metals, category 1 | H290—May be corrosive to metals | |
| Skin corrosion/irritation, category 1C | H314—Causes severe skin burns and eye damage | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the Future
Pedrolide1 is a diterpenoid that contains what had previously been an unprecedented 5–5–6–6–3 carbon skeleton. In 2021, Maria-José U. Ferreira* and collaborators at the University of Lisbon, the University of Szeged (Hungary), Uppsala University (Sweden), and the University of Porto (Portugal) isolated it from the flowering shrub Euphorbia pedroi that grows only in the Arrábida Natural Park in Portugal.
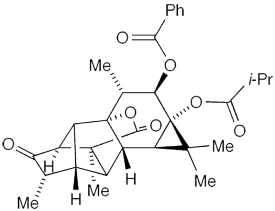
Ferreira et al. deduced its structure, proposed a possible biosynthetic pathway, and established that it, like other terpenoids in E. pedroi, exhibits multidrug-resistance reversal properties, which are crucial weapons in cancer treatments. This past April, Marlene Fadel and Erick M. Carreira* at ETH Zurich reported a 20-step enantioselective total synthesis of pedrolide.
1. CAS Reg. No. 2701553-64-0.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Angelic acid fast facts
| CAS Reg. No. | 565-63-9 |
| SciFinder nomenclature | 2-Butenoic acid, 2-methyl-, (2Z)- |
| Empirical formula | C5H8O2 |
| Molar mass | 100.21 g/mol |
| Appearance | White crystals or powder or colorless liquid |
| Melting point | 45 °C |
| Boiling point | 185 °C |
| Water solubility | Slight |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

