What molecule am I?
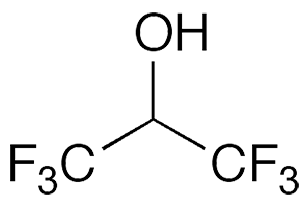
Hexafluoro-2-propanol (erroneously called “hexafluoroisopropanol”, abbreviated HFIP) is a low-boiling solvent used in a variety of chemical applications. It first appeared in the chemical literature in a 1960 Swiss patent to Soviet Union chemists Ivan L. Knunyants and M. P. Krasuskaya, who synthesized it by reducing hexafluoroacetone1 with sodium borohydride.
After 40 years or so of being considered a laboratory curiosity, HFIP began to receive chemists’ attention. A 2017 perspective by Ignacio Colomer, Timothy J. Donohoe, and co-workers at the University of Oxford (UK) described HFIP as a highly versatile solvent. Then, in 2022, Hashim F. Motiwala, Jeffrey Aubé, and colleagues at the University of North Carolina (Chapel Hill and Wilmington) reviewed the use of HFIP in organic synthesis. They described HFIP as a polar, strongly hydrogen bond–donating solvent with the “ability to stabilize ionic species, transfer protons, and engage in a range of other intermolecular interactions”.
After describing the physical properties of HFIP and comparing it with common solvents, Motiwala, Aubé et al. discussed the advantages of using it in 20 types of organic reactions, including solvolysis, carbon–carbon bond formation, carbon–hydrogen functionalization, condensation, and cyclization. The article includes an extraordinary 629 reaction schemes in which HFIP is used as the solvent, or in some cases as a reactant.
Not to be outdone, Colomer, Donohue et al. followed up their original article by describing HFIP as “more than a polar protic solvent”. In addition to covering the same ground as Motiwala, Aubé et al., they delved into its use in electrochemical methods, organometallic and inorganic chemistry, biology, and materials and polymer science. Both author sets predict that HFIP will continue to garner many more applications in multiple scientific fields.
1. CAS Reg. No. 684-16-2.
Hexafluoro-2-propanol hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 1A | H314—Causes severe skin burns and eye damage | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Reproductive toxicity, category 2 | H361—Suspected of damaging fertility or the unborn child | |
| Specific target organ toxicity, repeated exposure, category 2 | H373—Causes damage to organs through prolonged or repeated exposure | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
Aikinite1, a mineral with the empirical formula PbCuBiS3, was discovered in the Ural Mountains of Russia in 1843. It has since been found in Sweden, Australia (Tasmania), the United States (Idaho), and other countries. It is named after British geologist Arthur Aikin, even though he did not discover it.
This past April, Paz Vaqueiro at the University of Reading (UK), Marco Fornari at Central Michigan University (Mount Pleasant), and colleagues reported their study of the basis of aikinite’s extremely low thermal conductivity of 0.48 W/(m∙K) at 300 °C. According to the authors, data obtained from inelastic neutron scattering, neutron diffraction, and molecular dynamics simulations showed that the coupling of the motions of the Cu+, Pb2+, and Bi3+ cations “is an effective mechanism to achieve ultralow thermal conductivity in crystalline materials.”
trans-Nerolidol2 is a sesquiterpene alcohol found in essential oils from many flowering plants, including shrubs in the myrtle family (Myrtaceae), and legumes in the Fabaceae (aka Leguminosae) family. Its cis isomer is also found in nature. Each of the positional isomers exists as (+)- and (–)-stereoisomers. trans-Nerolidol has been known since at least 1923, when 1939 chemistry Nobelist Leopold Ružička at ETH Zurich synthesized the racemic mixture in three steps from geranyl chloride3.
The nerolidols are widely used as aroma and flavoring agents in foods, cosmetics, and personal-care products; but their supply is limited because the main sources are from plant extraction, which is costly and gives products of inconsistent purity. This May, Congqiang Zhang and co-workers at the Singapore Institute of Food and Biotechnology Innovation reported a biobased synthesis of trans-nerolidol in which the enzyme strawberry nerolidol synthase was engineered to produce the pure compound from sugars and glycerol in yields as high as 26%.
1. CAS Reg. No. 12232-84-7.
2. CAS Reg. No. 40716-66-3 (racemic).
3. CAS Reg. No. 5389-87-7.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Hexafluoro-2-propanol
fast facts
| CAS Reg. No. | 920-66-1 |
| SciFinder nomenclature | 2-Propanol, 1,1,1,3,3,3-hexafluoro- |
| Empirical formula | C3H2F6O |
| Molar mass | 168.04 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 59 °C |
| Water solubility | Miscible |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

