What molecule am I?

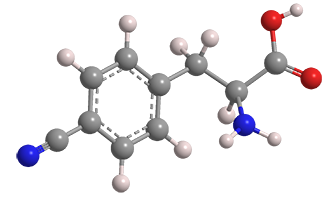
p-Cyano-l-phenylalanine (pCNPhe) is an unnatural amino acid used in optical probes for imaging proteins. It was introduced in a 1994 world patent application by Richard T. Dean and co-inventors at Diatide (Londonderry, NH), who used it as a chelator for the radionuclide technetium-99m in a procedure for detecting blood clots (thrombi). The patent application was followed by a 1996 Journal of Medicinal Chemistry article.
Since that finding, pCNPhe showed its versatility in other applications. In a 2009 report, Daniel P. Raleigh, Isaac Carrico, and coauthors at the State University of New York at Stony Brook and Franklin & Marshall College (Lancaster, PA) used the molecule as a fluorescence, infrared (IR), and Förster resonance energy transfer (FRET) probe to study protein folding, protein−membrane interactions, protein−protein interactions, and amyloid formation. They concluded that FRET, in particular, can be used directly to probe conformational changes in proteins.
In 2019, Megan C. Thielges and colleagues at Indiana University (Bloomington and Indianapolis) described the use of pCNPhe as an IR probe to characterize protein dynamics with high spatial and temporal resolution. They applied the probe at six sites in a Src1 homology 3 domain and found that their method revealed a wide range of microenvironments and distinct responses to ligand binding.
This past November, Paul S. Nerenberg, Christine M. Phillips-Piro, Scott H. Brewer, and collaborators at California State University, Los Angeles, and Franklin & Marshall once again demonstrated the utility of pCNPhe, this time as a “vibrational reporter” in the model system superfolder green fluorescent protein (sfGFP). They combined temperature-dependent IR spectroscopy, X-ray crystallography, and molecular dynamics simulations to investigate the local environment of the nitrile group in the protein. In one instance, they found that an interior site in sfGFP consists of three distinct local environments around the nitrile group, including nonspecific van der Waals interactions, hydrogen bonding to a structural water, and hydrogen bonding to a histidine side chain.
1. A proto-oncogene tyrosine–protein kinase.
p-Cyano-L-phenylalanine hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity-single exposure, respiratory system, category 3 | H335—May cause respiratory irritation | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules in the News
Calcium fluoride1 (CaF2), which occurs in nature as the mineral fluorspar, is the only commercial source of the world’s fluorine. The traditional way to extract fluorine from fluorspar is to treat the mineral with concentrated sulfuric acid, which liberates hydrogen fluoride (HF) gas. But HF is extremely toxic, corrosive, and difficult to handle.
This past month, a potentially practical and safer alternative to converting CaF2 to a less hazardous substance was reported. Simon Aldridge, Michael A. Hayward, Véronique Gouverneur, and collaborators at the University of Oxford (UK) and other institutions found that grinding fluorspar with crystalline dipotassium hydrogen phosphate2 (K2HPO4) gives a combination of tripotassium fluoride phosphate3 (K3HPO4F) and mixed potassium–calcium fluorophosphate–phosphate salts, which they call Fluromix. The authors showed that Fluromix can be used for the direct synthesis of compounds with carbon–fluorine and sulfur–fluorine bonds.
1. CAS Reg. No. 7789-75-5.
2. CAS Reg. No. 7758-11-4.
3. CAS Reg. No. 2943017-49-8.
Molecules in the News
MOTW highlights molecules that appear in major news outlets. See this week's edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
p-Cyano-L-phenylalanine
fast facts
| CAS Reg. No. | 167479-78-9 |
| SciFinder nomenclature | L-Phenylalanine, 4-cyano- |
| Empirical formula | C10H10N2O2 |
| Molar mass | 190.20 g/mol |
| Appearance | White crystals or powder |
| Melting point | Not reported |
| Water solubility | 100 g/L (est.) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

