What molecule am I?
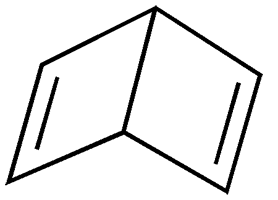
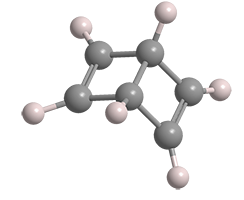
Earlier this month, Molecule of the Week brought you the venerable and extremely hazardous molecule benzene. This week’s molecule has one of the structures that was originally proposed for benzene: bicyclo[2.2.0]hexa-2,5-diene.
In 1869, chemist/physicist James Dewar at Cambridge University (UK) suggested the bicyclohexadiene as a possible benzene structure, although he eventually supported the correct structure as proposed by August Kekulé. Dewar’s structure became so well known that it is called Dewar benzene to this day.1
Almost a century later, Eugene E. van Tamelen* and Socrates P. Pappas at the University of Wisconsin (Madison) reported the synthesis of Dewar benzene. They began with cis-1,2-dihydrophthalic anhydride, which they irradiated with UV light to rearrange it to bicyclo[2.2.0]hexa-5-ene-2,3-dicarboxylic acid anhydride. Oxidative decarboxylation of the anhydride with lead tetraacetate gave the desired product in 20% yield. Spectroscopic observations and chemical conversions confirmed the Dewar benzene structure.
Dewar benzene is somewhat unstable; it has high strain energy and rearranges to benzene at ambient temperature with a half-life of ≈2 days. It has not been greatly studied, with fewer than 200 mentions in the chemical literature. It is also not an article of commerce; hence, no hazard information is available. Its hazardous properties are likely similar to those of the structurally related 1,4-cyclohexadiene (see table).
1. Chemists also know Dewar as the inventor of the vacuum flask that bears his name.
Dewar benzene hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 2 | H225—Highly flammable liquid and vapor | |
| Germ cell mutagenicity, category 1B | H340—May cause genetic defects | |
| Carcinogenicity, category 1A | H350—May cause cancer | |
| Specific target organ toxicity (blood), repeated exposure, category 1 | H372—Causes damage to blood through prolonged or repeated exposure | |
Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
*Hazard information for Dewar benzene is unavailable. Data are given for 1,4-cyclohexadiene, CAS Reg. No. 628-41-1.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
N,N-Di-(2-ethylhexyl)isobutyramide1 (DEHiBA) is a dialkylated amide that was first described in 1987 by C. Musikas at the Nuclear Studies Center (Fontenay-aux-Roses, France) as an extractant for ions of actinide elements, particularly uranium. Tributyl phosphate is currently the extractant of choice for recycling uranium and plutonium from used nuclear fuel; but its shortcomings have led researchers to investigate non–phosphate-containing complexation agents. In April, Gabriel B. Hall and co-workers at Pacific Northwest National Laboratory (Richland, WA) reported that high concentrations (1.5 M) of DEHiBA in n-dodecane solvent efficiently extracts uranyl nitrate [UO2(NO3)2] and nitric acid from aqueous solutions into the organic medium.
Arsenic trisulfide2 (As2S3) is a water-insoluble yellow solid that is the main constituent of the mineral orpiment. Its uses range from glass manufacture, pigments, semiconductors, and photoconductors. This past April, Fréderique T. H. Broers and collaborators at the Rijksmuseum (Amsterdam) and other institutions in the Netherlands, Belgium, and the United States explained the degradation of As2S3 pigments in artworks.
The authors elucidated two distinct degradation pathways: When irradiated with UV or visible light, As2S3 oxidizes to white arsenolite (arsenic trioxide, As2O3); whereas, in the presence of a medium such as oil or egg yolk, it converts to water-soluble As(V) species. The two processes are independent of each other and can occur simultaneously.
1. CAS Reg. No. 112724-95-5.
2. CAS Reg. No. 1303-33-9.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Dewar benzene
fast facts
| CAS Reg. No. | 5649-95-6 |
| SciFinder nomenclature | Bicyclo[2.2.0]hexa-2,5-diene |
| Empirical formula | C6H6 |
| Molar mass | 78.11 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 103.5 °C (est.) |
| Water solubility | Slight (est.) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

