What molecule am I?
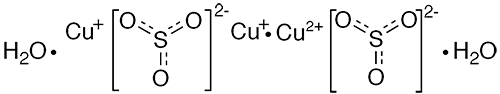
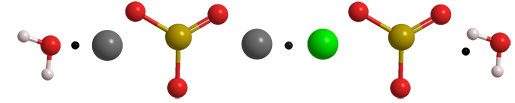
Chevreul’s salt is a double salt of copper(I) sulfite and copper(II) sulfite. It usually occurs as a dihydrate with the empirical formula Cu2SO3∙CuSO3∙2H2O. The color of its crystals and powder is often described in the literature as “brick red”. An anhydrous version of the salt1 also exists.
The salt was first prepared in 1812 by the prolific French chemist Michel Eugène Chevreul, whose work ranged from oils and fats to dyes to medicines. Although his eponymous salt is an inorganic compound, he is mainly known as a pioneer in organic chemistry; many biographical accounts of Chevreul, who lived to age 102, do not even mention the salt.
Chevreul’s salt was originally formed by heating an aqueous solution of copper(II) sulfate (CuSO4) and potassium metabisulfite (K2S2O5). The metabisulfite partially reduces Cu(II) to Cu(I) and displaces the sulfate with sulfite. The insoluble salt precipitates, leaving an acidic solution.
In 1999, Michiko B. Inoue and collaborators at the University of Sonora (Hermosillo, Mexico) and the University of Arizona (Tucson) described the preparation of Chevreul’s salt by heating aqueous CuSO4 with sodium hydrogen sulfite (NaHSO3) at 60–70 °C. They went on to measure the salt’s spectroscopic and magnetic properties. The following year, L. A. Silva, J. R. Matos, and J. B. de Andrade* at the Federal University of Bahia (Salvador, Brazil) and the University of São Paulo (Brazil) reported another synthesis in which an aqueous solution of CuSO4 is saturated with sulfur dioxide gas at ambient temperature. These authors prepared other Cu2SO3 double salts and determined their thermal properties.
Chevreul’s salt has few practical uses. A 1987 Polish patent described its use as a copper catalyst for making photopolymeric positive photoresists. In 2006, Turan Çalban*, Sabri Çolak, and Murat Yeşilyurt at Atatürk University (Erzurum, Turkey) demonstrated how dissolved copper extracted from the ore can be recovered by injecting sulfur dioxide and precipitating the salt.
1. CAS Reg. No. 15293-86-4.
Chevreul’s salt hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
*Hazard information for Chevreul’s salt is unavailable. Data are given for copper(I) sulfate, CAS Reg. No. 17599-81-4.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms
Molecules in the News
Staurosporine1 is a bisindole alkaloid antibiotic isolated in 1977 from the bacterium Streptomyces staurosporeus by Satoshi Ōmura2 and co-workers at Kitasato University (Tokyo). Its antibiotic properties have been studied since the 1980s; in recent years, it was discovered to have activity against some forms of cancer.
Last month, Tim Cernak and colleagues at the University of Michigan (Ann Arbor), along with collaborators at Abbvie (North Chicago, IL) and Merck (Kenilworth, NJ), reported the development of a “mini medicinal chemistry tool kit”, a high-throughput experimentation system that uses microliter quantities of material to run specified chemical reactions. They used the system to produce 46 analogues of staurosporine for further medical research.
Celastrol3, also called tripterine, is a pentacyclic nortriterpene quinone that is found in the roots of Tripterygium wilfordii and Tripterygium regelii, vines used in traditional eastern medicine. The blood-red compound was isolated from T. wilfordii in 1936 by T. Q. Chou* and P. F. Mei at the Institute of Materia Medica (Shanghai).
Celastrol has been shown to have antibacterial, antioxidant, anti-inflammatory, and anticancer activities. But medical research has concentrated on its antiobesity effects, as first demonstrated on overfed mice in 2015 by Umut Ozcan and coauthors at Harvard Medical School and Massachusetts General Hospital (both in Boston). The molecule is difficult to synthesize; but last month Søren Bak, Karel Miettinen, Sotirios C. Kampranis, and co-workers at the University of Copenhagen reported the elucidation of its complete biosynthetic route. Their work allows it to be biosynthesized in a yeast medium, catalyzed by the enzyme cytochrome P450.
1. CAS Reg. No. 62996-74-1.
2. Ōmura was a recipient of the 2015 Nobel Prize in Physiology or Medicine for his work on avermectins and ivermectins.
3. CAS Reg. No. 34157-83-0.
Molecules in the News
Molecule of the Week introduces a new feature in which we highlight molecules that appear from major news outlets. See the inaugural Molecules in the News below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Chevreul’s salt fast facts
| CAS Reg. No. | 13814-81–8 |
| SciFinder nomenclature | Sulfurous acid, copper(1+) copper(2+) salt (2:2:1), dihydrate |
| Empirical formula | H4Cu3O8S2 |
| Molar mass | 386.78 g/mol |
| Appearance | Dark red-orange crystals or powder |
| Melting point | >200 °C (dec.) |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

