What molecule am I?
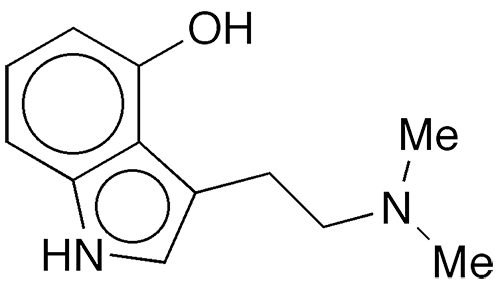
Psilocin, also called N,N-dimethyl-4-hydroxytryptamine, is a psychoactive substance that was first isolated in 1958 by Albert Hofmann and co-workers at Sandoz (Basel, Switzerland; now part of Novartis) from the psychedelic mushroom Psilocybe mexicana. The Hofmann group elucidated the structure of the molecule and synthesized it the same year.
Psilocybin, the Molecule of the Week for October 2, 2017, is the monophosphate ester of psilocin; it is more abundant in mushrooms than the parent compound. The tryptamine derivatives were concurrently isolated and synthesized.
In 1966, the United States passed laws that prohibited the production, trade, and ingestion of hallucinogenic drugs, including psilocin and psilocybin. Five years later, the United Nations listed them as Schedule I drugs1 under the Convention on Psychotropic Substances. Some countries, however, allow them to be used for religious purposes or in medical research.
In light of ongoing research on the use of psychedelics to treat neuropsychiatric disorders, David E. Olson, Pamela J. Lein, and colleagues at the University of California, Davis, sought to develop a screening method for determining the neurotoxicity of 13 such compounds, including psilocin and psilocybin. This past February, they described a study in which larval zebrafish were exposed to the drugs at various concentrations and observed teratological and behavioral abnormalities in the fish.
The researchers found that the tryptamines were less neurotoxic to zebrafish than LSD and psychostimulants (e.g., amphetamines). They concluded that their screening method could generate a robust database for rapidly quantifying neurotoxicity in psychedelics being developed as therapeutics.
For more information on psilocin and psilocybin, see the ScienceDirect topics page.
1. Drugs with a high potential for abuse.
Psilocin hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the Future
Orellanine1, officially called 3,3’,4,4’-tetrahydroxy-2,2’-bipyridine-1,1’-dioxide, is a mycotoxin that occurs in mushrooms of the genus Cortinarius that grow worldwide. Orellanine first came to light in 1952, when 11 people died after eating poisonous mushrooms. In the early 1960s, Polish physician Stanisław Grzymala isolated the compound from C. orellanus, identified it, and measured its nephrotoxicity to various mammals. Its structure was confirmed in 1979 by Wieław Z. Antkowiak and Wiesł P. Gessner at Adam Mickiewicz University (Poznań, Poland).
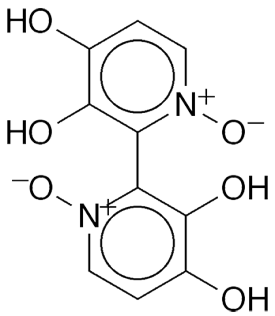
Since its discovery, orellanine was cited in the deaths of numerous people who ingested Cortinarius spp. that they mistook for edible mushrooms. But just last month, in a review of the biochemistry of orellanine, Mark J. Lyons, Carsten Ehrhardt, and John J. Walsh* at Trinity College Dublin (Ireland) reported that the toxin could have an upside. The pharmaceutical researchers cited promising preclinical studies of orellanine against metastatic clear-cell renal cell carcinoma. Oncorena (Lund, Sweden) began Phase 1 and 2 clinical trials against the disease in 2022.
1. CAS Reg. No. 37338-80-0.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Psilocin fast facts
| CAS Reg. No. | 520-53-6 |
| SciFinder nomenclature | 1H-Indol-4-ol, 3-[2-(dimethylamino)ethyl]- |
| Empirical formula | C12H16N2O |
| Molar mass | 204.27 g/mol |
| Appearance | White crystals or solid |
| Melting point | 174.5 °C |
| Water solubility | 4 g/L |
MOTW update
Coronene1 was the Molecule of the Week for April 10, 2006. It is a polynuclear aromatic compound sometimes called “superbenzene”. It is considered a pollutant in Earth’s atmosphere, but it has value as an ultraviolet phosphor. Coronene was detected in carbon stars in 1995.
Earlier this month, Patrick Hemberger, Alexander M. Mebel, Ralf I. Kaiser, and collaborators at Paul Scherrer Institute (Villigen, Switzerland), Florida International University (Miami), and the University of Hawaii at Manoa (Honolulu) reported that molecular-beam experiments and electronic structure calculations provided evidence that coronene is formed in carbon stars via a complex series of gas-phase reactions.
1. CAS Reg. No. 191-07-1.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

