What molecule am I?
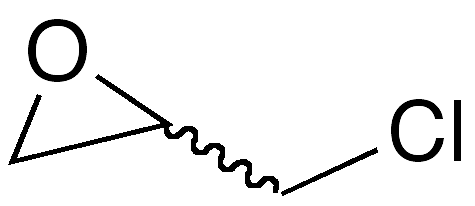
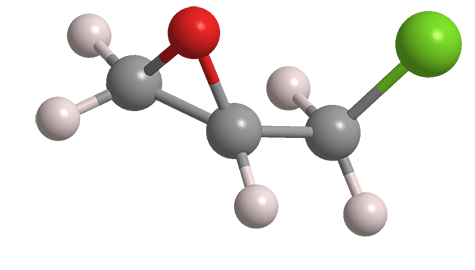
Epichlorohydrin, aka 2-(chloromethyl)oxirane, is a hazardous bifunctional liquid with a chloroform-like odor. The article of commerce is normally a racemic mixture of its two enantiomers; the 3-D image shown here is the (+)-isomer1.
In 1848, the pioneering French chemist Marcellin Berthelot—at the age of 21—was the first to describe epichlorohydrin. He prepared it by treating glycerol with hydrogen chloride gas. Berthelot’s synthesis has since been refined and is the basis of the modern manufacturing process. An alternative method is the epoxidation of allyl chloride. Current worldwide production is estimated to be >2 million tonnes.
By far the largest use of epichlorohydrin is in the production of epoxy resins. It is most often combined with bisphenol A in a base-catalyzed condensation reaction to produce the resin bisphenol A diglycidyl ether2. It is also used as a reagent for chemical and polymer synthesis and as a solvent for resins and coatings.
The health hazards associated with epichlorohydrin, bisphenol A, and epoxy resins require the use of protective equipment and adequate ventilation.
1. CAS Reg. No. 67843-74-7.
2. CAS Reg. No. 1675-54-3.
(±)-Epichlorohydrin hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 3 | H226—Flammable liquid and vapor | |
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Acute toxicity, dermal, category 3 | H311—Toxic in contact with skin | |
| Skin corrosion/irritation, category 1B | H314—Causes severe skin burns and eye damage | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Carcinogenicity, category 1A | H350—May cause cancer | |
Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
*Compilation of two safety data sheets; other data sheets list additional hazards.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
Harmaline1 (4,9-dihydro-7-methoxy-1-methyl-3H-pyrido[3,4-b]indole) is a fluorescent indole alkaloid that was first isolated in 1841 by F. Goebel of Dorpat (now Tartu), Estonia, from the seeds of Peganum harmala, or Syrian rue, a Mediterranean herb. It has since been found in other plants. It is a central nervous system inhibitor and an amine oxidase inhibitor that is psychoactive in humans and prohibited or controlled in some countries.
This past April, Adela M. Sánchez-Moreiras and collaborators at the University of Vigo (Spain) and the University of Milan (Italy) reported that harmaline may be a useful natural herbicide. In a search for herbicides with modes of action different from current ones to which weeds have developed resistance, the researchers used the common weed Arabidopsis thaliana as a model target to test the potency of harmaline, which had shown phytotoxicity in earlier studies. They demonstrated that harmaline is phytotoxic to adult A. thaliana plants at concentrations as low as 14 μM.
Morin2 [2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one] is a flavonol and natural yellow dye that was isolated in 1895 by Arthur George Perkin* and Frank Pate at Yorkshire College (now the University of Leeds, UK) from the heartwood of the jack-fruit tree (Artocarpus integrifolia). The same year, the Perkin–Pate group and Charles S. Boyer of Camden, NJ, isolated it from other trees, including old fustic (Maclura tinctoria).
Chemists synthesized morin as early as 1906; but its value in dyeing and electroplating—and as a potential anticancer agent—called for improved production methods. In April, Thomas Lindel and coauthors at the Technical University of Braunschweig and ORSATec (Bobigen, both in Germany) devised an inexpensive synthesis of morin that does not require chromatographic separation of the product. Starting from phloroglucinol3 and 2,4-dimethoxybenzaldehyde4, they produced morin on a 40-g scale in 18% overall yield.
1. CAS Reg. No. 304-21-2.
2. CAS Reg. No. 480-16-0.
3. CAS Reg. No. 108-73-6.
4. CAS Reg. No. 613-45-6 .
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
(±)-Epichlorohydrin
fast facts
| CAS Reg. No. | 106-89-8 (racemic) |
| SciFinder nomenclature | Oxirane, 2-(chloromethyl)- |
| Empirical formula | C3H5ClO |
| Molar mass | 92.52 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 118 °C |
| Water solubility | 60 g/L (20 °C) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

