What molecule am I?
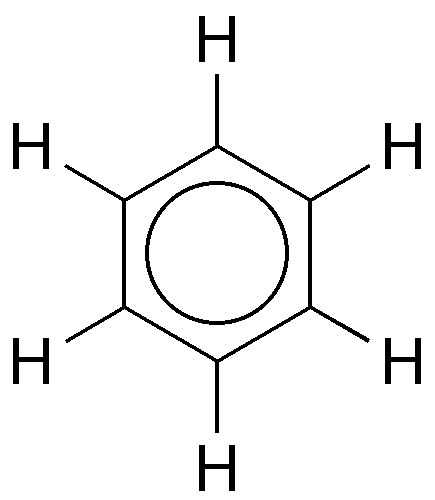
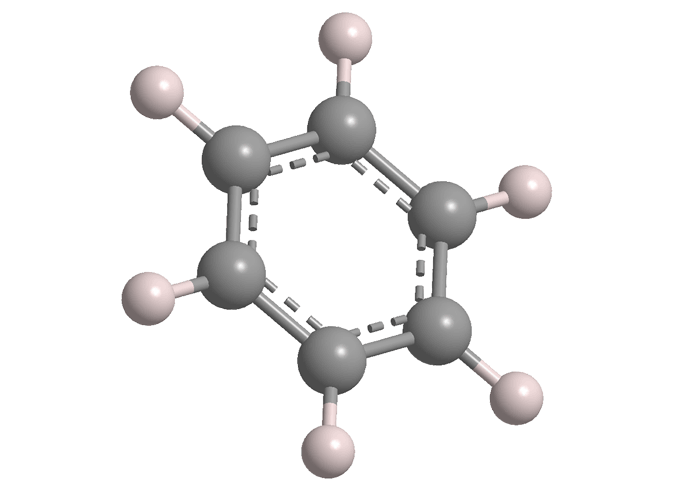
Benzene is the cornerstone of aromatic organic molecules. It has a long and checkered history—much too long to cover here—so the focus will be on the very old and very new.
Legendary British scientist Michael Faraday is primarily known for his discoveries in electricity and electromagnetism; but it was he who first isolated benzene in 1825 from coal-derived “illuminating gas”, which was used for lighting in the early 19th century.
Chemists soon determined that the benzene molecule contains six carbon and six hydrogen atoms; but for decades, they struggled to determine its precise structure. In 1865, German chemist Friedrich August Kekulé published a paper in which he described benzene as consisting of a ring of six carbon atoms, each bonded to a hydrogen atom. The story goes that Kekulé dreamt of a snake biting its tail, which inspired him to conceive of the structure.
Kekulé’s original concept was that the carbon atoms are attached to each other by alternating single and double bonds. But in the years following his original paper, his experiments showed that all six bonds are equivalent, which meant that each one oscillates between single and double bonding. Eventually, researchers, including Linus Pauling, concluded that this phenomenon is the basis of aromaticity, in which the carbon atoms are connected to each other via σ- and π-bonding rather than discrete double bonds.
In the 20th century, benzene, derived mostly from petroleum, came into widespread production for use as a solvent and as a starting material for manufacturing other organic compounds, especially ethylbenzene, which is converted to styrene. The use of benzene for making a large array of products continues today; but its use as a solvent declined abruptly in the 1980s, when it was established as a human carcinogen.
People nonetheless continue to be exposed to benzene. Gasoline contains 0.5–2.0 vol% benzene, which is one reason that gasoline dispensers now connect tightly to automobiles’ fuel tanks. In the past year, studies have shown that household stoves that use natural gas or propane can expose residents to harmful concentrations of benzene.
Last month, Robert B. Jackson at Stanford University (CA) and colleagues there and at other institutions in the San Francisco Bay area reported that gas and propane combustion from stoves emits benzene and increases indoor air pollution. They reported that the mean benzene emissions from gas and propane burners set on high and ovens set to 350 °F [177 °C] ranged from 2.8 to 6.5 μg/min, 10–25 times higher than emissions from electric coil and radiant alternatives.
Jackson et al.’s findings verify others’ reports that gas stovetops and ovens could be health hazards. The issue has become political: Some jurisdictions have already banned new installations of gas-fueled cooking equipment; in other areas, protesters are loudly crying, “Save our gas stoves!”
Benzene hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 2 | H225—Highly flammable liquid and vapor | |
| Aspiration hazard, category 1 | H304—May be fatal if swallowed and enters airways | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Germ cell mutagenicity, category 1B | H340—May cause genetic defects | |
| Carcinogenicity, category 1A | H350—May cause cancer | |
| Specific target organ toxicity, repeated exposure, category 1 | H372—Causes damage to blood through prolonged or repeated exposure | |
| Short-term (acute) aquatic hazard, category 2 | H401—Toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 3 | H412—Harmful to aquatic life with long-lasting effects | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
L-Methionine1 is a sulfur-containing essential amino acid. In 1921, pathologist John Howard Mueller at Columbia University (New York City) discovered it in streptococci cultures and found that it contributed to bacterial growth. Seven years later, George Barger* and Frederick Philip Coyne at the University of Edinburgh determined its structure and synthesized it.
Ever since this early work, the biosynthesis, biochemistry, and nutritional aspects of proteinogenic methionine have been thoroughly investigated. Although methionine is generally considered to be beneficial in the diet, this past March, Guowei Le and colleagues at Jiangnan University (Wuxi, China) and other Chinese universities reported that restricting methionine in the diet of middle-aged mice improves glucose metabolism by alleviating pancreatic apoptosis, thus promoting insulin secretion.
Cannabichromene2 (CBC) is a minor nonpsychoactive component of marijuana (Cannabis sativa). It may contribute to the analgesic effect of cannabis and has been suspected of enhancing the psychoactivity of Δ9-tetrahydrocannabinol3 (Δ9-THC).
Both enantiomers of CBC are found in C. sativa; the abundance of CBC and the relative amounts of each enantiomer depend on the specific strain of the plant. This April, Giulia Mazzoccanti, Giovanni Appendino, and colleagues at the Sapienza University of Rome and other Italian institutions described their study of the formation of CBC in several C. sativa cultivars. CBC concentrations are greater in the leaves of young plants; because of the potent synergistic activity of CBC with Δ9-THC, the authors emphasized the desirability of cultivating strains that retain this juvenile metabolic trait.
1. CAS Reg. No. 63-68-3.
2. CAS Reg. No. 20675-51-8.
3. CAS Reg. No. 1972-08-3.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
Benzene fast facts
| CAS Reg. No. | 71-43-2 |
| SciFinder nomenclature | Benzene |
| Empirical formula | C6H6 |
| Molar mass | 78.11 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 80 °C |
| Water solubility | 1.8 g/L (20 °C) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

