What molecule am I?
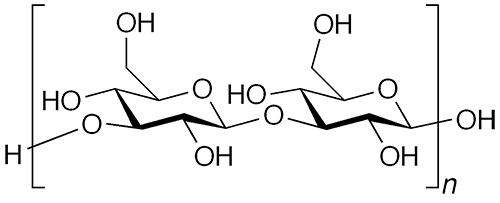
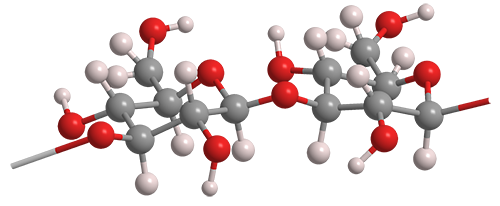
Paramylon is a glucose polymer similar to cellulose, but they differ in how the glucose (or glucan) units are linked. Cellulose contains β-1→4 glucan linkages, whereas in paramylon, the glucan linkages are in the β-1→3 configuration.
Paramylon is found in microalgae of the genus Euglena, single-cell flagellate eukaryotes that grow in salt- and freshwater. Botanist Bastiaan J. D. Meeuse at Delft University of Technology (the Netherlands) did early work on the characterization of paramylon. As reported in a 1965 article, Meeuse and co-workers isolated the molecule from E. spirogyra by comminuting it in a blender, suspending the particles in water, shaking the suspension in butyl alcohol, and isolating pure paramylon granules from the aqueous layer.
The 1→3 bonding in paramylon allows the polymer to self-organize into a triple helix, as illustrated in the image at the right. Paramylon molecules extracted from Euglena spp. tend to have very uniform chain lengths.
Materials chemist Motonari Shibakami at the National Institute of Advanced Industrial Science and Technology (AIST, Tsukuba, Japan) is developing paramylon-based materials for potential commercialization. The materials include nanofibers, thermoplastic films, optical lenses, fibers, and adhesives. These applications have small carbon footprints compared with fossil feedstock sources and are cost-effective because of the ease of cultivating Euglena organisms and extracting paramylon from them.
For more information about paramylon processing see a review by Eric Leroy and coauthors at the University of Nantes (Carquefou, France) and the University of Reims Champagne–Ardenne (Reims, France).
MOTW updates
Adenosine1 and inosine2 were the Molecules of the Week for June 5, 2010, and July 15, 2019, respectively. They are nucleosides that differ only by the hydroxyl configurations on the ribofuranose ring. In 2019, researchers found that a gene editor unexpectedly converted a small amount of adenosine in an RNA to inosine.
Earlier this month, Eli Eisenberg and collaborators at Tel Aviv University, the University of Connecticut Health Center (Farmington), Bar-Ilan University (Ramat Gan, Israel), and the University of Puerto Rico (San Juan) reported that this conversion can be advantageous to squids and other cephalopods. The researchers found that seawater temperature decreases induce the cephalopods to self-edit mRNA adenosine to inosine. The nucleoside transformation enables the animals to prevent low-temperature “brain freeze”.
Tetrachloroethylene3 was the Molecule of the Week for December 18, 2017. Also known as perchloroethylene or “perc”, it is a nonflammable solvent widely used in dry cleaning. It is also a suspected carcinogen that has come under scrutiny by the US Environmental Protection Agency.
This month, EPA announced that it is proceeding to restrict the use of tetrachloroethylene. It proposed a rule to phase it out from all consumer uses in 2 years and from dry-cleaning uses within 10 years. This was after a 2020 agency finding that the solvent poses health risks, such as neurological, kidney, liver, and immunological effects.
1. CAS Reg. No. 58-61-7.
2. CAS Reg. No. 58-63-9.
3. CAS Reg. No. 127-18-4.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
.
Paramylon fast facts
| CAS Reg. No. | 51052-65-4 |
| SciFinder nomenclature | Paramylon |
| Empirical formula | (C6H10O5)n |
| Molar mass | ≈270 kg/mol |
| Appearance | White solid or powder |
| Melting point | Does not melt |
| Water solubility | Insoluble |
Paramylon hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.