What molecule am I?
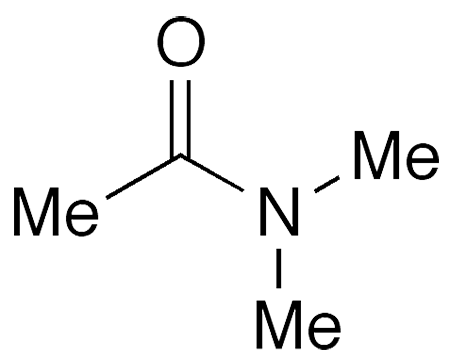
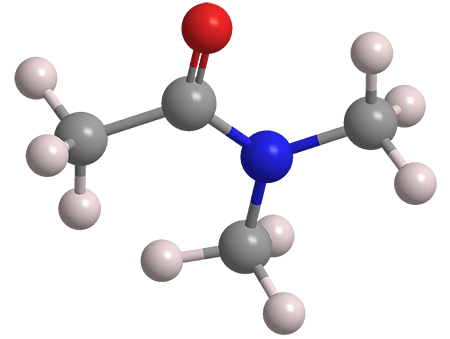
N,N−Dimethylacetamide (DMAc) is a dipolar, aprotic, high-boiling, oily solvent and reagent. It is miscible with water and most oxygen- and nitrogen-containing organic solvents. It has a faint ammonia odor.
DMAc traces its roots at least as far back as 1914, when Marc Tiffeneau* and K. Fuhrer at Hôpital Boucicaut1 (Paris) identified it as a product of heating N,N-dimethylbenzylamine2 and acetic anhydride3 to 200 °C in a sealed tube. Then, in 1931, in a treatise on the synthesis of aliphatic amides, James A. Mitchell and E. Emmet Reid at Johns Hopkins University (Baltimore) described preparing DMAc in 84% yield by heating acetic acid and dimethylamine4 at 150 °C for 3 h. This, along with using acetic anhydride or methyl acetate5 instead of the acid, is the process for manufacturing DMAc today.
DMAc is used as a solvent for producing fibers and for synthesizing organic compounds, notably pharmaceuticals. Its broad range of miscibility makes it useful in mixed solvents. It is also stable to strong bases; but it hydrolyzes in the presence of acids. As the hazard information table shows, it must be handled with care.
For more information on DMAc, see the ScienceDirect topics page.
1. Now part of Hôpital Européen Georges-Pompidou.
2. CAS Reg. No. 103-83-3.
3. CAS Reg. No. 108-24-7.
4. CAS Reg. No. 124-40-3.
5. CAS Reg. No. 79-20-9
N,N−Dimethylacetamide hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 4 | H227—Combustible liquid | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
| Eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity, single exposure; narcotic effects, category 3 | H336—May cause drowsiness or dizziness | |
| Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
| Reproductive toxicity, category 1B | H360—May damage fertility or the unborn child | |
| Specific target organ toxicity, repeated exposure, category 1 | H372—Causes damage to liver through prolonged or repeated exposure | |
| Specific target organ toxicity, repeated exposure, category 2 | H373—May cause damage to respiratory system through prolonged or repeated exposure | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
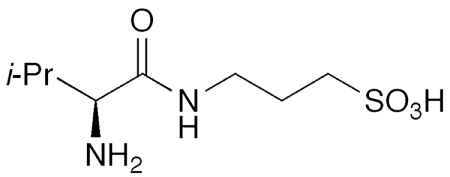
ALZ-801 was first described in a 2008 US patent application by Neurochem (International)2 (Vaud, Switzerland). The compounds of the invention were claimed to be prodrugs that delivered 3-amino-1-propanesulfonic acid3 (aka tramiprosate) to treat nervous system disorders, including Alzheimer’s. When Alzheon was formed in 2013, the company acquired the rights to develop ALZ-801.
The US Food and Drug Administration designated ALS-801 as a fast-track drug in 2017. This past December, Alzheon announced the completion of the enrollment of oral ALZ-801 in a Phase 3 clinical trial to evaluate its action against apolipoprotein E4/4 homozygotes in early Alzheimer’s disease. Individuals with the apolipoprotein E gene represent ≈15% of all Alzheimer’s patients. If the trial is successful, the company anticipates filing a new drug application in 2024.
1. CAS Reg. No. 1034190-08-3.
2. Neurochem (International) changed its name to BELLUS Health the same year and is now owned by GlaxoSmithKline.
3. CAS Reg. No. 3687-18-1.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
N,N−Dimethylacetamide
fast facts
| CAS Reg. No. | 127-19-5 |
| SciFinder nomenclature | Acetamide, N,N-dimethyl- |
| Empirical formula | C4H9NO |
| Molar mass | 87.12 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 165–166 °C |
| Water solubility | Miscible |
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
MOTW update
Xenon difluoride1 (XeF2) was the Molecule of the Week for February 7, 2011. A white crystalline solid with an unpleasant odor, in 1962 it became one of the first compounds to be prepared from “inert” gases. Since then, it has been widely studied and incorporated into larger molecules. Earlier this month, Gary J. Schrobilgen and co-workers at McMaster University (Hamilton, ON) reported the synthesis of coordination complexes of XeF2 with the bromyl cation2 (BrO2+), such as [BrO2(XeF2)2][SbF6]. The authors described the properties of the complexes, including their coordination chemistry.
1. CAS Reg. No. 13709-36-9.
2. CAS Reg. No. 58409-45-3.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

