What molecule am I?
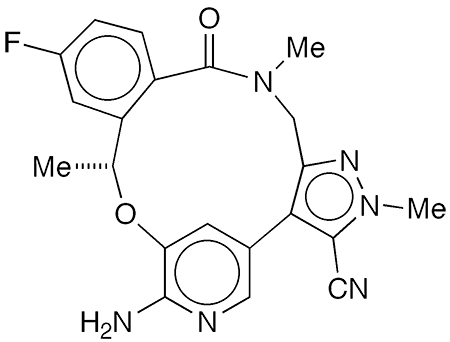
(R)-Lorlatinib1 is an oral medication for treating anaplastic lymphoma kinase (ALK)–positive non–small-cell lung carcinomas resistant to crizotinib2, an earlier drug used to combat the cancer. It was first disclosed by Pfizer (New York City) in a 2013 world patent application.
In 2014, Ted W. Johnson, Paul F. Richardson, and colleagues at Pfizer Worldwide Research and Development (San Diego) described the discovery of what was then called PF-06463922. They used structure-based drug design, lipophilic efficiency considerations, and physical-property–based optimization procedures to synthesize potent macrocyclic ALK inhibitors with the desired characteristics for effective carcinoma control. The inhibitors were potent against wild-type ALK and clinically reported ALK domain mutations. PF-06463922 emerged as the optimum drug candidate.
The US Food and Drug Administration granted orphan status to lorlatinib in 2014 and full approval in 2018 for second- or third-line treatment of ALK-positive metastatic non-small-cell-lung-cancer. The European Medicines Agency followed suit in 2019. The following year, NPS MedicineWise, an Australian medical information provider, issued a fact sheet for lorlatinib.
1. SciFinder: 2H-4,8-methenopyrazolo[4,3-h][2,5,11]benzoxadiazacyclotetradecine-3-carbonitrile, 7-amino-12-fluoro-10,15,16,17-tetrahydro-2,10,16-trimethyl-15-oxo-, (10R)-.
2. CAS Reg. No. 877399-52-5.
(R)-Lorlatinib hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Germ cell mutagenicity, category 2 | H341—Suspected of causing genetic defects | |
| Specific target organ toxicity, liver and pancreas, repeated exposure, category 2 | H373—May cause damage to the liver and pancreas through prolonged or repeated exposure | |
*This information is from one safety data sheet; other data sheets state that it is not a hazardous substance or mixture.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
In 2002, A. Douglas Kinghorn and coauthors at the University of Illinois at Chicago and the Ministry of Health of Peru (Lima) isolated several constituents of the roots of Renealmia nicolaioides, a South American plant used in traditional medicine. Among the isolates were three new prenylated dihydrochalcones, (±)-nicolaioidesins A, B, and C1. Since then, the nicolaioidesins have been investigated as potential drug candidates or precursors.
In March this year, Suresh Awale and co-workers at the University of Toyama (Japan) reported that (+)-nicolaioidesin C (Nic-C) exhibited antitumor activity in vitro and in vivo in a pancreatic cancer mouse model. Nic-C is called an “antiausterity agent” because it eliminates cancer cells’ ability to adapt to nutrition deprivation, thus limiting the cells’ migration in the body.
(−)-Ambrox2, or ambroxide, is the most prominent aroma component of ambergris, a waxy substance produced by sperm whales (Physeter macrocephalus). It is widely used in perfumes; but because sperm whaling is now banned, it must be manufactured.
In March, Eric Eichhorn and Fridtjof Schroeder at Givaudan Schweiz (Kemptthal, Switzerland) disclosed a sustainable method for producing (−)-ambrox. They began with the commercially available fermentation product (E)-β-farnesene3 and converted it in one chemical step and one enzymatic step to the desired compound. The authors compared their method with other routes to (−)-ambrox.
1. CAS Reg. Nos. 475579-25-0, -26-1, and -27-2, respectively.
2. CAS Reg. No. 6790-58-5.
3. CAS Reg. No. 18794-84-8.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
(R)-Lorlatinib
fast facts
| CAS Reg. No. | 1454846-35-5 |
| Empirical formula | C21H19FN6O2 |
| Molar mass | 406.41 g/mol |
| Appearance | White crystals or powder |
| Melting point | 184–187 °C |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

