What molecule am I?
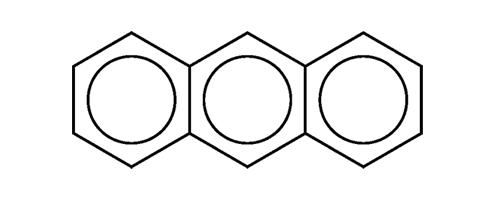
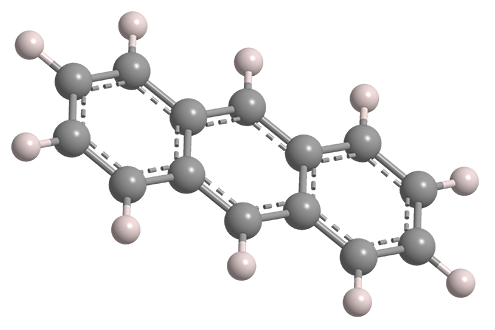
Anthracene is one of the smaller polynuclear aromatic hydrocarbons. (The only smaller one is naphthalene.) It was isolated from coal tar in 1832 by pioneering French chemists Jean-Baptiste Dumas and Auguste Laurent.
Anthracene can be synthesized by the Elbs reaction, in which o-tolyl phenyl ketone is dehydrated at 400–450 ºC. But most commercial anthracene is still recovered from coal tar.
In commerce, anthracene is mainly used as a starting material for the manufacture of 9,10-anthraquinone, which in turn is used to make colorants such as the red dye alizarin. More recently, crystalline anthracene was found to be a useful wide band-gap semiconductor in devices such as organic field-effect transistors and scintillators for detecting high-energy subatomic particles.
Anthracene hazard information*
| Hazard class** | Hazard statement | |
|---|---|---|
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Carcinogenicity, category 1A | H350—May cause cancer | |
| Hazardous to the aquatic environment, acute hazard, category 1 | H400—Very toxic to aquatic life | |
| Hazardous to the aquatic environment, long-term hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Combined from multiple safety data sheets.
**Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Anthracene fast facts
| CAS Reg. No. | 120-12-7 |
| SciFinder nomenclature | Anthracene |
| Empirical formula | C14H10 |
| Molar mass | 178.23 g/mol |
| Appearance | White crystals or powder |
| Melting point | 218 ºC |
| Water solubility | 44 μg/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

