What molecule am I?
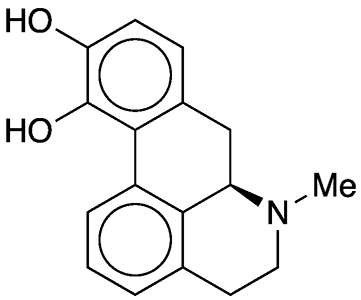
Apomorphine is a synthetic opioid that has been known since at least 1871, when German researcher E. L. Mayer treated the natural drug morphine with zinc chloride in hydrochloric acid solution. About 70 years later, Lyndon Small*, Burt F. Faris, and James Mallonee at the University of Virginia (Charlottesville) elucidated the mechanism of this reaction (without ZnCl2, which is apparently unnecessary).1
In 1973, John L. Neumeyer and colleagues at Arthur D. Little (Cambridge, MA) and Sterling-Winthrop Research Institute (New York) reported the total synthesis of racemic apomorphine and other morphine and codeine derivatives. Eight years later, Vishnu J. Ram and Neumeyer*, now at Northeastern University (Boston) synthesized both apomorphine enantiomers from the natural opiates thebaine2 and bulbocapnine3.
Unlike, many opioids, apomorphine has several medical uses not related pain management. In humans, it has such diverse applications as a Parkinson’s disease drug, as an emetic, and most recently, as a treatment for erectile dysfunction. It has also been touted as a treatment for heroin addiction, but no clinical evidence has shown that it is effective for this purpose.
Apomorphine can be used as an emetic for dogs when they ingest poisonous substances such as antifreeze. It must, however, be injected because it travels too slowly through the blood–brain barrier when given orally.
Apomorphine is somewhat unstable; it decomposes and turns green when it is exposed to light and air. It is much more stable as its hydrochloride hemihydrate4, which is the preferred article of commerce.
1. More than 80 years ago, authorities were concerned about opiate addiction. The authors noted, “The work reported in this paper is part of a unification of effort by a number of agencies having responsibility for the solution of the problem of drug addiction.”
2. CAS Reg. No. 115-37-7.
3. CAS Reg. No. 298-45-3.
4. CAS Reg. No. 41372-20-7.
Apomorphine hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Sensitization, respiratory, category 1 | H334—May cause allergy or asthma symptoms or breathing difficulties if inhaled | |
*Information for the more common article of commerce, apomorphine hydrochloride hemihydrate.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW update
1,4-Benzoquinone1 (aka quinone) was the Molecule of the Week for November 23, 2009. It is a useful oxidizing agent and, along with its reduced form, hydroquinone2 (1,4-benzenediol), is the basis of many redox systems.
This month, Qinglei Meng, Buxing Han, and collaborators at the Chinese Academy of Sciences (Beijing) and East China Normal University (Shanghai) reported an innovative electrochemical method for simultaneously producing benzoquinone and cyclohexanone3 from phenol and water. In the authors’ electrochemical system, the cathode consists of nickel and platinum supported on nitrogen-doped hierarchically porous carbon (NHPC), whereas the anode contains iron and ruthenium, also supported on NHPC. Benzophenone and cyclohexanone form at the cathode and anode, respectively, both with >99.9% selectivity.
1. CAS Reg. No. 106-51-4.
2. CAS Reg. No. 123-31-9.
3. CAS Reg. No. 108-94-1.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Apomorphine fast facts
| CAS Reg. No. | 58-00-4 |
| SciFinder nomenclature | 4H-Dibenzo[de,g] quinoline-10,11-diol, 5,6,6a,7-tetrahydro-6-methyl-, (6aR)- |
| Empirical formula | C17H17NO2 |
| Molar mass | 267.32 g/mol |
| Appearance | White crystals or powder |
| Melting point | 195 °C (dec.) |
| Water solubility | 20 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

