What molecules are we?
Artemisinin is a venerable malaria drug that was introduced more than 40 years ago. It was the Molecule of the Week for December 12, 2005.
Artemisinin is an excellent remedy for malaria. For many years, however, its supply has been unpredictable. The sole source of the drug is the sweet wormwood plant (Artemisia annua) that grows in Southeast Asia; but its production cycle is vicious. Farmers grow the plant only when prices are high; the inevitable oversupply lowers prices and production drops, raising prices again.
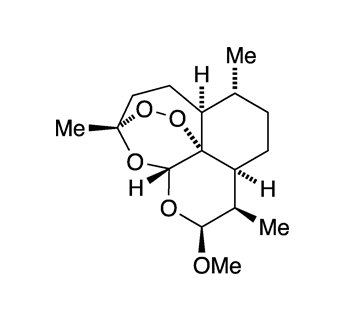
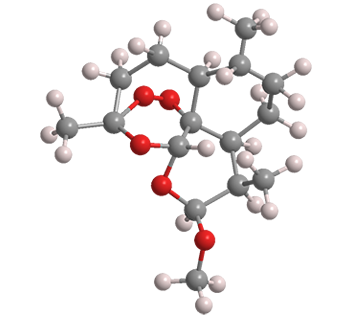
As a result, medicinal researchers sought to synthesize an artemisinin precursor that would ensure a constant supply of the drug at lower cost than agricultural artemisinin. Sanofi (Gentilly, France) developed a synthesis of artemether (Figure 1), which it commercialized in 2013. Unfortunately, the project failed because the semisynthetic artemisinin could not be produced less expensively than the farmed product.
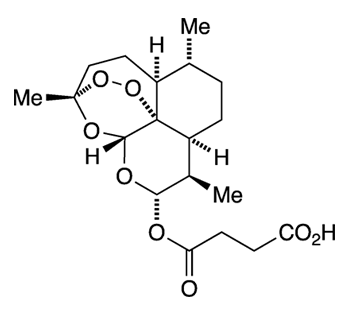
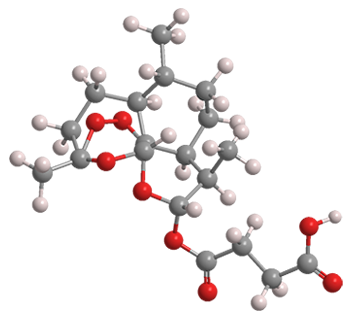
Since then, drug researchers concentrated on improving the yield of artemisinin and its derivatives from A. annua. For example, the leaves of the plant contain as much dihydroartemisinic acid (DHAA) as artemisinin. Earlier this year, Kerry Gilmore and Peter H. Seeberger at Max Planck Institute of Colloids and Interfaces (Potsdam, Germany) reported that crude extracts of A. annua can be fed into a photochemical flow reactor to produce artemisinin, artemether, and a third active pharmaceutical ingredient, artesunate (Figure 2).
Artesunate turns out to be a particularly attractive malaria drug. It is much more water-soluble than artemisinin, so it can be taken by mouth* or by injection. It is safe and well-tolerated by adults and children. Its success has earned it a place in the World Health Organization's List of Essential Medicines.
*But overdoses can be harmful; see hazard information table.
Artemether hazard information
| GHS classification**: acute toxicity,oral, category 4 | |
| H302—Harmful if swallowed | |
**Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Artesunate hazard information
| GHS classification: acute toxicity,oral, category 4 | |
| H302—Harmful if swallowed | |
| GHS classification: acute toxicity,dermal, category 4 | |
| H312—Harmful in contact with skin | |
| GHS classification: acute toxicity,inhalation, category 4 | |
| H332—Harmful if inhaled | |
Artemether fast facts
| CAS Reg. No. | 71963-77-4 |
| Molar mass | 298.37 g/mol |
| Empirical formula | C16H26O5 |
| Appearance | White to pale yellow crystals or powder |
| Melting point | 86–90 ºC |
| Water solubility | 12 mg/L (est.) |
Artesunate fast facts
| CAS Reg. No. | 88495-63-0 |
| Molar mass | 384.42 g/mol |
| Empirical formula | C19H28O8 |
| Appearance | White crystals or powder |
| Melting point | 140–142 ºC |
| Water solubility | 680 mg/L |
MOTW update:
July 1, 2024
Artemether1 is a precursor to the malaria drug artemisinin2 that was developed in the 2010s; but it was abandoned because it is more expensive than natural artemisinin.
In June, Qi-qun Tang at Fudan University (Shanghai) and coauthors there and at other Chinese universities described a potential new use for artemether. They discovered that the compound could be the first effective treatment for polycystic ovary syndrome (PCOS), a widespread endocrine disorder that affects ≈10% of reproductive-age women. In a pilot clinical study of 19 women with PCOS, most of the subjects exhibited fewer symptoms of the syndrome and had more regular menstrual cycles.
1. CAS Reg. No. 71963-77-4.
2. CAS Reg. No. 63968-64-9.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

