What molecule am I?
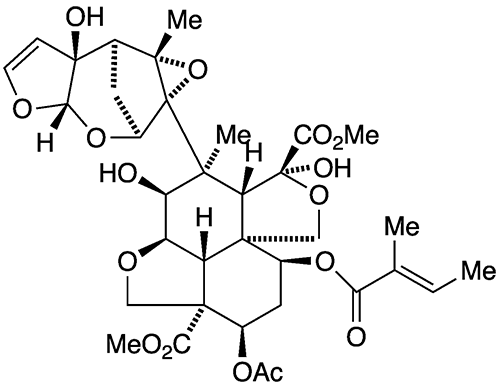
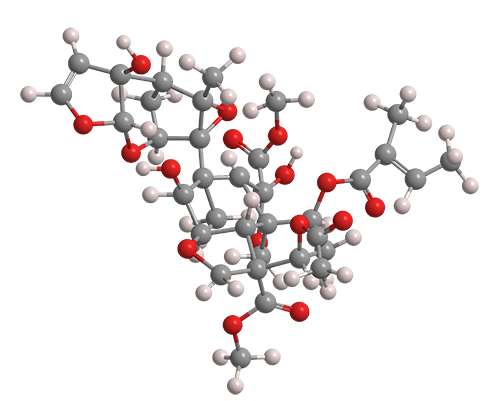
Azadirachtin is an impressive molecule. It contains eight rings (five of which incorporate oxygen atoms), 16 chiral centers, and three quaternary carbon atoms. Its functionalities include a hydroxyl group, an enol ether, an acetal, two hemiacetals, an epoxide, and four carboxylic acid esters.
Synthetic organic chemists had a tough time synthesizing azadirachtin, but it came naturally to the neem tree (Azadirachta indica), a member of the mahogany family that grows in India, Iran, and parts of Africa. Azadirachtin, along with many related compounds, is found in neem seeds, leaves, and bark. Its biosynthetic pathway has not been completely elucidated.
For centuries, the seeds and leaves of A. indica have been known to repel harmful insects from agricultural crops. In the 1960s, chemists began to study neem seed extracts to determine the active ingredients. J. H. Butterworth and E. D. Morgan at the University of Keele (UK) identified azadirachtin as the key molecule in 1968. Stephen V. Ley and four co-workers at the University of Cambridge (UK) completed its prodigious total synthesis in 2007.
Azadirachtin is a white microcrystalline solid with a strong garlicky or sulfurous odor, which is a bit strange because it contains no sulfur. Perhaps the odor is what repels insects. In any case, neem oil (extracted from the seeds) is used in many commercial pesticides and growth regulators. Although azadirachtin has some toxic properties (see hazard information table), it is harmless to mammals in the amounts normally used. As the table also shows, however, it brings some environmental concerns.
Azadirachtin has another, perhaps unexpected, use. Much of the Indian population chews neem twigs or uses them as brushes to clean their teeth. Taking things further, neem bark, leaves, and oil are used in many “natural” toothpastes. You can purchase all of these products in health food stores or online.
Azadirachtin hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Acute toxicity, dermal, category 5 | H313—May be harmful in contact with skin | |
| Carcinogenicity, category 1B | H350—May cause cancer | |
| Hazardous to the aquatic environment, acute hazard, category 2 | H401—Toxic to aquatic life | |
| Hazardous to the aquatic environment, long-term hazard, category 2 | H411—Toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Azadirachtin fast facts
| CAS Reg. No. | 11141-17-6 |
| SciFinder nomenclature | 1H,7H-Naphtho[1,8- bc:4,4a-c′]difuran -5,10a(8H)- dicarboxylic acid, 10-(acetyloxy)octahydro -3,5-dihydroxy -4-methyl-8-{[(2E)-2-methyl-1-oxo-2-buten-1-yl]oxy}-4-[(1aR,2S,3aS, 6aS,7S,7aS)-3a,6a,7,7a-tetrahydro-6a- hydroxy-7a-methyl-2,7-methanofuro[2,3-b]oxireno[e]oxepin -1a(2H)-yl]-, 5,10a- dimethyl ester, (2aR,3S,4S,4aR, 5S,7aS,8S,10R, 10aS,10bR)- |
| Empirical formula | C35H44O16 |
| Molar mass | 720.71 g/mol |
| Appearance | White microcrystalline solid |
| Melting point | 165 ºC |
| Water solubility | 0.26 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

