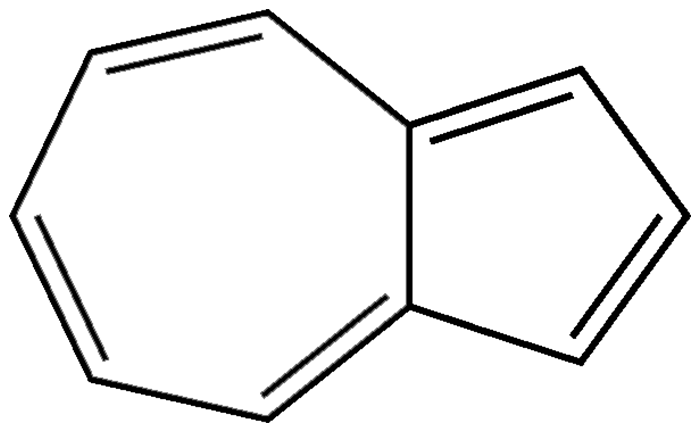
Azulene (pronounced “as you lean”) is an aromatic hydrocarbon that contains no six-membered rings. It is an isomer of naphthalene and has a similar odor, but instead of white, its crystals are dark blue. (Azul is the Spanish word for blue.)
Blue-colored compounds with the azulene structure have been known for six centuries. Azulene itself was synthesized from octahydronaphthalene by B. Susz, A. S. Pfau, and P. A. Plattner in 1937 and from indane by Plattner and G. Magyar in 1942.
Azulene’s 10–π-electron system qualifies it as an aromatic compound. Similarly to aromatics that contain benzene rings, it undergoes reactions such as Friedel–Crafts substitutions. And like the cyclopentadienyl anion and the cycloheptatrienyl cation, it forms π-complexes with metals such as molybdenum and iron.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

