What molecule am I?

Borneol is a chiral bicyclic alcohol that exists in nature as two enantiomers, (–)- or L-borneol (shown) and (+)- or D-borneol1. Along with former Molecules of the Week eucalyptol (aka 1,8-cineole), camphor, and α-pinene, (–)-borneol is a principal component of the oil and leaves of the herb rosemary (Salvia rosmarinus, formerly Rosmarinus officinalis).
Borneol was known as early as 1842, when French chemist Charles Frédéric Gerhardt identified it and named it “camphre de Bornéo”, or “Borneo camphor”. In the 1870s, English chemist Henry E. Armstrong and others measured the properties and reactions of borneol (which he called “camphol”) and related compounds. The 19th century chemists did not realize that borneol existed in two isomers and their publications are listed under racemic borneol2.
Borneol can be oxidized to camphor by treating it with sodium hypochlorite and other oxidizing agents. But when camphor is reduced to borneol (e.g., with sodium borohydride), the main product is borneol’s positional isomer isoborneol3. As seen in the fast facts table, (–)-borneol’s melting and boiling temperatures are very close to each other. As a result, when the compound is heated up to ≈207 °C, it melts and sublimes.
All of which brings us back to rosemary, which you might use to season stuffing and vegetables for your Thanksgiving dinner. According to S. Kokkini, R. Karousou, and E. Hanlidou at Aristotle University (Thessaloniki, Greece) in the second edition of the Encyclopedia of Food Sciences and Nutrition, “[Rosemary oils] with a decreased amount of α-pinene and camphor and increased amounts of 1,8-cineole and borneol are judged to be of a better quality.”
For more information on rosemary and borneol, see the ScienceDirect rosemary topic page.
1. CAS Reg. No. 464-43-7.
2. CAS Reg. No. 507-70-0.
3. CAS Reg. No. 124-76-5 (racemic).
(–)-Borneol hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable solids, category 1 | H228—Flammable solid | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
Long-term (chronic) aquatic hazard, category 3 | H412—Harmful to aquatic life with long-lasting effects | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the Future
Explosives chemists are always looking to make materials that are safer to handle and have lower environmental impact. The current trend is toward organic compounds that contain several nitrogen atoms; these substances are targeted to replace heavy metal–containing explosives such as lead azide1 [Pb(N3)2].
Last March, Hongwei Yang, Chuan Xiao, Guangbin Cheng, and colleagues at Nanjing University of Science and Technology (China) and China Northern Industries Group (Nanjing), reported the synthesis and properties of DTAT-K2, a [5,6,5]-tricyclic bistetrazole molecule (see image) that could replace lead azide as a “green” primary explosive with good priming ability.
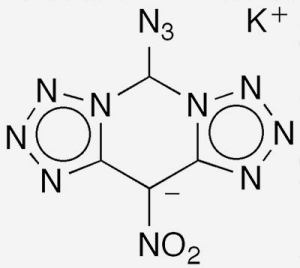
DTAT-K, with the chemical name 5-azido-10-nitro-bis(tetrazolo)[1,5-c:5′,1′-f]pyrimidine, potassium salt, was synthesized from commercially available, inexpensive 4,6-dichloro-5-nitropyrimidine3 by treating it with sodium azide and then replacing the sodium ion with potassium. The authors’ discovery was somewhat serendipitous because they only expected the reaction to displace the chlorine atoms on the pyrimidine ring with azide groups. The formation of the tricyclic molecule and the addition of a third azide moiety were a big surprise.
1. CAS Reg. No. 13424-46-9.
2. CAS Reg. No. 2918805-26-0.
3, CAS Reg. No. 4316-93-2.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
(–)-Borneol fast facts
| CAS Reg. No. | 464-45-9 |
| SciFinder nomenclature | Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, (1S,2R,4S)- |
| Empirical formula | C10H18O |
| Molar mass | 154.25 g/mol |
| Appearance | White crystals, powder, or solid |
| Melting point | 207–209 °C |
| Boiling point | 210–212 °C |
| Water solubility | 0.74 g/L (25 °C) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

