What molecule am I?
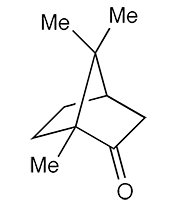
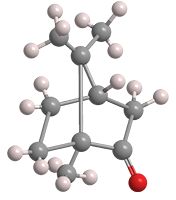
Camphor is a naturally occurring terpene found in trees in the laurel family (Lauraceae), notably camphor laurel, or Cinnamomum camphora, that is native to east and south Asia and now grows worldwide. It has been used in folk medicine for centuries.
Camphor can exist in two enantiomers: (+)- or (R)-camphor (shown), the predominant natural isomer, and (–)-or (S)-camphor1, which occurs in sand sage (Artemisia filifolia), a flowering plant native to the western United States. Synthetic camphor is usually a racemic mixture2 of the two enantiomers.
Camphor, with its familiar, penetrating odor, has a wide range of uses, including as a plasticizer for modified celluloses; in lacquers, varnishes, and plastics; as a moth repellent alternative to p-dichlorobenzene; and as a preservative in cosmetics and embalming fluids. In medicine, it is an ingredient in topical creams and ointments to treat itching, irritation, and joint pain; and it is used internally to prevent or relieve gas in the gastrointestinal tract.
1. CAS Reg. No. 464-48-2.
2. CAS Reg. No. 76-22-2.
Camphor hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable solids, category 2 | H228—Flammable solid | |
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity, single exposure, lungs, category 2 | H371—May cause damage to lungs if inhaled | |
| Short-term (acute) aquatic hazard, category 2 | H401—Toxic to aquatic life | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW updates
Ammonia1 was the Molecule of the Week for February 8, 2021. A colorless, toxic gas, it is an important industrial and agricultural chemical and a starting material for making a host of other compounds. It was the topic of two literature reports in August.
The city of Beijing has chronically poor air quality. Yixin Guo, Lin Zhang, and coauthors at Peking University (Beijing) and other Chinese research institutions contend that the pollution may be even worse than previously thought. They found that summertime ammonia emissions are substantially underestimated in Beijing because the prevailing atmospheric models do not completely account for sources such as fertilizer, power plants, vehicle traffic, and residences.
In contrast, Ronny Neumann and collaborators at the Weizmann Institute of Science (Rehovot, Israel) and the University of Rovira i Virgili (Tarragona, Spain) developed a way to make ammonia from molecular nitrogen and water. This finding eliminates the coproduction of carbon dioxide, which occurs when ammonia is made from hydrogen derived from fossil fuel sources such as natural gas. The researchers’ electrolytic process is catalyzed by a tri-iron–substituted polyoxotungstate, {SiFe3W9}, in the presence of lithium or sodium cations. Water acts as both a proton and electron donor.
1. CAS Reg. No. 7664-41-7.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Camphor fast facts
| CAS Reg. No. | 464-49-3 |
| SciFinder nomenclature | Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1R,4R)- |
| Empirical formula | C10H16O |
| Molar mass | 152.23 g/mol |
| Appearance | White crystals |
| Melting point | 179 °C |
| Water solubility | 1.2 g/L (20 °C) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

