What molecule am I?
The architect Buckminster Fuller popularized geodesic domes in the late 1940s. The domes are structures that sustain their own weight and, despite their curved appearance, consist of planar equilateral triangles.
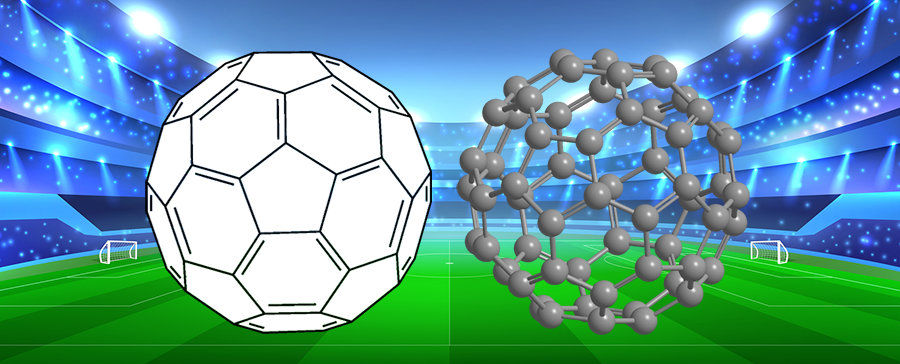
When chemists Harold W. Kroto at the University of Sussex (Falmer, UK); Robert F. Curl, Jr., and Richard E. Smalley at Rice University (Houston); and co-workers discovered the spherical C60 molecule in 1985, it was natural to name it buckminsterfullerene or, colloquially, “buckyballs”. Although Fuller’s domes consist only of triangles, C60 contains regular pentagons and hexagons.
The same researchers, who went on to win the 1996 Nobel Prize in Chemistry, synthesized other nonspherical fullerenes such as C70. Fullerenes also exist in nature, primarily in soot; but they have also been observed in outer space.
Buckminsterfullerene, like other carbon allotropes, is black. But, unlike other carbons, it is soluble in nonoxygenated organic solvents such as toluene and carbon disulfide. The solutions are purple, magenta, or green, depending on the concentration and solvent–solute interactions.
Many C60 derivatives have been prepared, some with functional groups that can participate in subsequent reactions or catalysis. Fullerenes also have applications in nanotechnology.
Buckminsterfullerene, of course, is noteworthy for its similarity to a soccer ball. So keep it in mind when you watch this year’s men’s FIFA World Cup competition, which begins on June 14.
Buckminsterfullerene
fast facts
| CAS Reg. No. | 99685-96-8 |
| Molar mass | 720.64 g/mol |
| Empirical formula | C60 |
| Appearance | Shiny black needle-like crystals |
| Melting point | ≈600 ºC (subl.) |
| Water solubility | Insoluble |
Buckminsterfullerene hazard information
| GHS classification*: serious eye irritation, category 2A | |
| H319—Causes serious eye irritation | |
| GHS classification: specific target organ toxicity, single exposure; respiratory tract irritation, category 3 | |
| H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

