What molecule am I?
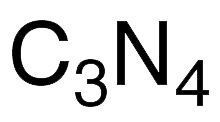
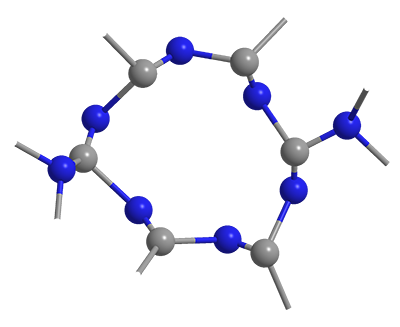
Carbon nitride (C3N4), like elemental carbon, has several stable allotropes. The two most common are β-C3N4 (partial structure shown), which is analogous to diamond, and g-C3N4, whose properties are more like graphite.
In the late 1980s, theoretical physicists Amy Y. Liu at the University of California, Berkeley, and Marvin L. Cohen at Lawrence Berkeley Laboratory predicted that β-C3N4 would have short, strong bonds and could be harder than diamond. The material proved to be difficult to synthesize; but in 1993, Chunming Niu, Yuan Z. Lu, and Charles M. Lieber* at Harvard University (Cambridge, MA) created thin films of β-C3N4 using pulsed laser ablation of graphite targets combined with an intense atomic nitrogen source.
Also in 1993, Wolfgang Schnick at the University of Bayreuth (Germany) reviewed the theoretical and synthetic literature on β-C3N4; and, like Liu and Cohen, he surmised that the substance would be harder than diamond. But 30 years later, this prediction has not been demonstrated. It has been shown, however, that the bulk modulus (resistance to compression) of β-C3N4 is similar to that of diamond.
g-C3N4, as might be expected, consists largely of planar molecular units, mostly heptazines (condensed s-triazines). It usually contains a small amount of hydrogen at the peripheries of these structures. In 2008, Markus Antonietti and coauthors at the Max Planck Institute of Colloids and Interfaces (Golm, Germany) and the Fritz Haber-Institute of the Max Planck-Society (Berlin) reported that g-C3N4 can be made by polymerizing small C–N molecules such as cyanamide, dicyandiamide, and melamine. The researchers showed that the graphitic compound’s semiconductor properties give it “unexpected” catalytic activity for a variety of organic reactions.
Subsequently, several research teams found that g-C3N4 is a powerful photocatalyst. Much of this work is summarized in excerpts from the 2021 book Materials Science in Photocatalysis. If you want to learn even more, read “A Tour-Guide through Carbon Nitride-Land” (2021) by Vincent Wing-hei Lau at Korea University (Seoul) and Bettina V. Lotsch at the Max Planck Institute for Solid State Research (Stuttgart, Germany).
Graphitic carbon nitride hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the Future
Oteseconazole1 is a fluorinated tetrazole derivative used to treat chronic vaginal yeast infections. The US Food and Drug Administration approved it under the trade name Vivjoa in April 2022. According to the manufacturer, Mycovia Pharmaceuticals (Durham, NC), it is the only FDA-approved medication for this condition. Mycovia is also in Phase 2 studies on oteseconazole for treating onychomycosis, a fungal nail infection.
1. CAS Reg. No. 1340593-59-0.

Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Carbon nitride fast facts
| CAS Reg. No. | 143334-20-7 |
| SciFinder nomenclature | Carbon nitride |
| Empirical formula | C3N4 |
| Molar mass | 92.06 g/mol |
| Appearance | Off-white to yellowish-brown crystals or powder |
| Melting point | β-C3N4: 2950 °C g-C3N4: 600–700 °C (dec.) |
| Water solubility | Insoluble |
MOTW update
Sulfoxaflor1 was the Molecule of the Week for October 5, 2015. It is an insecticide that the US Environmental Protection Agency approved for use in 2013. In 2015, EPA banned its use because of its toxicity to honeybees; but, controversially, in 2019, under the Trump administration, the agency reauthorized its use on several crops. This past December, the US Court of Appeals for the 9th Circuit ruled that the EPA broke the law by reauthorizing sulfoxaflor because it failed to assess endangered species risks and did not give the public a chance to comment on its decision.
1. CAS Reg. No. 946578-00-3.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

